It is an anion in which carbon is having unshared pair of electrons and has a negative charge with the three substituents for the total of eight valance electrons. It is found in the trigonal pyramidal geometry. A carbanion is formally the conjugate base of the carbon acid. In the organic chemistry, it is one of the reactive intermediates. It is nucleophile whose reactivity and stability is determined by the various factors such as the inductive effect, extent of the conjugation of the anion, polarizabilities, and hybridization of the charge bearing atom. But the general and easiest way for the determination of the carbanion stability is the fact that the effects which increase the negative charge density of the carbon atom of the carbanion decrease the stability of the carbanion. If one substituent increases the negative charge density on the carbon atom, then the whole carbanion is destabilized.
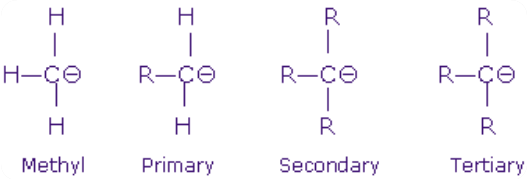
Carbanions are named after their parent alkyl group. They are also called as the primary, secondary and tertiary and it is determined by the nature of the carbon atom bearing the negative charge. The state of negatively charged carbon atom is of sp3 hybridization and these hybrid orbitals are directed to the corners of the tetrahedron. Three hybrid orbitals take part to make the single covalent bond with the other atoms. They make a pyramidal structure like the NH3 molecule. They are highly reactive intermediates.
In carbon anion carbon is trivalent, it forms three bonds, and has a formal negative charge at least in the one significant mesomeric contributor or resonance form. A carbanion is the member of the organic compounds and the negative electric charge is predominantly located on the carbon atom. Formally, they are derived from the organic molecules by removing the positively charged atom or the group of atoms and they are of significant importance as chemical intermediates which are used for the preparation of the other substances. It has many industrial applications and is used to make plastic and many other useful things.
Methide is the simplest carbanion that is derived from the methane that is an organic compound by the loss of the proton. While studying the structures of the carbanions it is important to distinguish between the localized and the delocalized ions. In the localized ions, negative charge is confined to the one carbon atom whereas in the delocalized ions negative charge is distributed over the various atoms. If carbanions are present in the solution, then there must be a corresponding cation in the same solution and as a result, the covalent bond is formed.
As carbanion is the reactive intermediate so it is encountered in the organic chemistry for instance in the organometallic chemistry. Carbanion does exist in stable forms as well. Any molecule that contains the carbon and hydrogen can become carbanion by losing the proton. Any hydrogen that contains the carbon and hydrogen bonds can be considered as an acid but with the corresponding Pka value. There is strong evidence that carbanion can be chiral as well especially in the certain, organolithium compounds.