The thermal decomposition of materials at elevated temperatures in an inert atmosphere is known as Pyrolysis. It is an irreversible process and involves a change of chemical composition. The word Pyrolysis comes from the Greek-derived elements pyro “fire” and lysis “separating”.
Pyrolysis can be rightly defined as the conversion of a compound into smaller fragments in the absence of air through the application of heat. Basically it involves the chemical breakdown of compounds, which contain carbon, using heat. For Pyrolysis to happen, high pressure and temperature above 430 °C (800 °F) is required. Normally it is used for organic things. It is different from combustion reactions as it happens in the absence of air. Because of this oxidation of compounds does not take place. Pyrolysis of alkanes is generally also termed as cracking.
Pyrolysis Process
In the absence of air when Alkane vapours are passed through red-hot metal it breaks down into simpler hydrocarbons. This process requires both high temperatures and high pressure. It takes place without a catalyst. The presence of a catalyst such as platinum or palladium is required for this reaction at low temperatures and pressure. Generally we obtain the large hydrocarbon during the process of fractional distillation of crude oil/ Petroleum. In cracking, the hydrocarbon molecules randomly break into smaller hydrocarbon compounds. Carbon-carbon double bondsare present in some compounds obtained from cracking.
Some amounts of oxygen, water, or other substances may be present in some setting. This is done so that combustion, hydrolysis, or other chemical processes may occur besides pyrolysis proper. Sometimes those chemical are added intentionally. This can be seen using many processes like in the burning of firewood, in the traditional manufacture of charcoal, and in the steam cracking of crude oil.
Also, the starting material may be heated in an inert atmosphere or in vacuum to avoid adverse chemical reactions. Pyrolysis when done in a vacuum lowers the boiling point of the by-products, improving their recovery.
The factors responsible for the formation of products during cracking are:
- Nature of alkane
- Temperature and pressure
- Presence or absence of a catalyst
Pyrolysis of Alkanes
The rate of pyrolysis increases with the increase in the molecular weight and branching in an alkane in contrast to what happens in combustion,. During the fission of C-C bonds, alkanes and alkenes are produced, whereas the fission of C-H bond results in alkene and hydrogen. C-H bond fission takes place due to the catalytic action of Cr2O3, V2O2, MoO3 and C-C bond fission which occurs under the presence of SiO2, Al2O3, and ZnO.
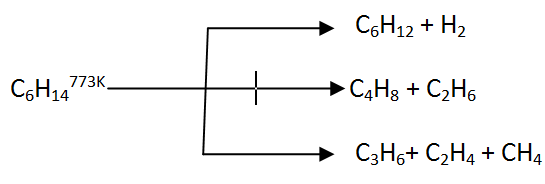
Cracking of alkanes follows a free radical mechanism. It plays an important role in the petroleum industry. By cracking, the higher molecules of alkanes are transformed into lower molecules (petrol C6 to C11)
For example, Dodecane, which is a component of kerosene oil, gives a mixture of heptane and pentane as pyrolysis products. This is obtained on heating it to a temperature of 973K under the catalytic action of platinum, palladium or nickel.
C12H26−→−−−−pt/pd/ni C7H16 + C5H10 + other products