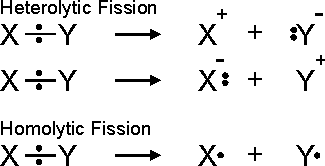
Organic chemistry – Homolytic fission and Heterolytic Fission of a covalent bond
The Organic reaction usually involve making and breaking of covalent bonds. The fission of covalent bonds can take place in two ways:
- Homolytic fission
- Heterolytic fission
Homolytic fission:
In Homolytic fission, the cleavage of the covalent bond takes place in such a way that each bonded atom retains one electron of the shared pair. This is symmetrical fission and leads to the formation of neutral species having unpaired electrons. These species are called free radicals. The free radicals are denoted by putting a dot over the symbol of atom or group of atoms. The single electron movement is shown by half-headed curved arrows.,
Heterolytic fission:
Heterolytic fission is unsymmetrical. In this, the covalent bond is broken in such a way that one of the fragments takes both the electron of the pair leaving none on the other. This results into two charged particles. One has the sextet of electrons hence possess a positive charge, while the other has a complete octet of electrons with at least one lone pair of electron thus possesses a negative charge. The species that have sextet of electrons and have a positive charge is called carbocation also carbonium ion. The other one who has a lone pair of electron and complete octet of electrons having a negative charge is called carbanion.
Now let us talk a bit about each one of the species we get by both of this fission’s:
Free radicals:
A free radical can be defined as an atom or groups of atoms having an unpaired electron. These are produced during the homolytic fission of a covalent bond.
These free radicals are very reactive. This is because of the fact that they have a strong tendency to pair up their unpaired electron with another electron from wherever available. These are very short-lived and occur only as a reaction intermediate. For example, dissociation of chlorine gas in the presence of ultraviolet light produces chlorine free radicals as shown below:
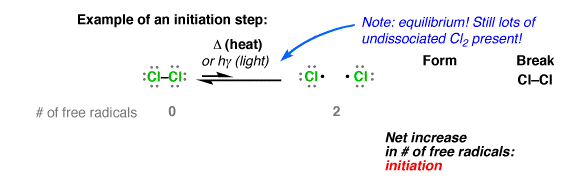
Stability of free radicals: the order of stability of alkyl free radical is:
This order of stability can be explained on the basis of hyperconjugation. Larger the number of alkyl groups attached to the carbon atom carrying the odd electron, greater is the delocalization of odd electron and hence more stable is the free radical.
Carbocation:
It is defined as a group of atoms that contain positively charged carbon atom having only six electrons in its valence shell. Earlier carbocation was known as a carbonium ion. They are obtained by the heterolytic fission of a covalent bond. The carbocations are also classified as primary, secondary, tertiary depending upon one, two, or three carbon atom is attached to the carbon atom bearing the positive charge as shown below:
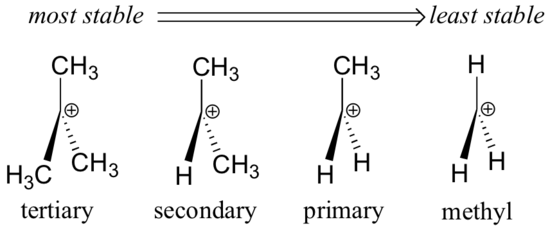
The Relative stability of carbocation:
We know that the methyl group has a +ve inductive effect means it is electron-donating group. The alkyl group attached to the positively charged carbon atom tends to release electrons towards carbon. Because of this positive inductive effect there occurs the decrease the +ve charge on the carbon atom but itself becomes somewhat positive. Hence, the positive charge on the carbon atom gets dispersed. The dispersal of positive charge results into stability, hence more the alkyl groups attached to the positively charged carbon atom more stable is the carbocation.
Thus, the relative stability is
Carbanion:
A carbanion may be defined as a species containing a carbon atom carrying a negative charge and possessing eight electrons in its valence shell. These are generated by the heterolytic fission of a covalent bond involving carbon atom in which the atom linked to the carbon goes without the bonding electrons. As a result of this, carbon acquires a negative charge. When a group attached to carbon atom leaves without electron pair, methyl carbanion is formed.
Like carbocation carbanions are also classified as primary, secondary and tertiary.
The order of stability of carbanion is reverse of carbocation as shown below:
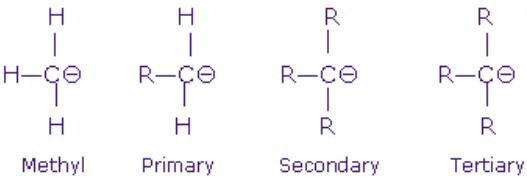
This can be explained by +I inductive effect. Alky group have positive I-effect so they release electrons and increase the density on negatively charged carbon hence makes it more unstable. Hence, more the alkyl group attached to the negatively charged carbon atom more unstable is the carbanion.