Chemical Kinetics: Activation Energy
Molecules are required to possess a minimum amount of energy to undergo a reaction. In order to form products, bonds must be broken in the reactants. Bond breakage requires energy. Molecules that move too slowly, with low kinetic energy, don’t react when they collide. Activation energy, Ea, is the minimum energy needed to begin a chemical reaction. Ea will vary with the type of reaction.
Consider the rearrangement of methyl isonitrile:
In H3C-NC, theCN≡Cbond keeps bending until the CN bond breaks down and the N≡C portion is perpendicular to the H3C portion. The structure formed is called an activated complex that is in a transition state. The energy required for the twist and break is defined as the activation energy, Ea. Once the C-N bond breaks, the N≡C portion continues to rotate forming a C-C≡N bond.
Fig 1: Reaction pathway for an activated complex.
Visualisation of energy changes throughout a process can illustrate the rearrangement of methyl isonitrile in a helpful manner. The change in energy, ∆E, for the reaction is the difference in energy between CH3N≡C and CH3CN.The high point on the diagram above is the transition state. The species that are present in the transition state is called the activated complex. And, the energy gap between the reactants and the activated complex is denoted as the activation energy barrier. The activation energy, Ea, is the difference in energy between reactants, CH3N≡C, and the transition state. The rate depends on Ea. If the “hill” is taller, the reaction rate is slower. If the “hill” is shorter the rate is faster.
For instance, if a forward reaction is exothermic (CH3N≡C CH3CN), then the reverse reaction is endothermic (CH3CN CH3N≡C).The methyl isonitrile molecule gains enough energy so that it can overcome the activation energy barrier. Recall that as temperature increases, the KEtotal increases and the number of molecules with energy greater than Ea increases. Soif the temperature is high enough, the reaction can make it “over the hill” and proceed. Likewise, it does not matter whether a reaction is exothermic or endothermic. Both reactions need to overcome the activation energy in order to initiate the reaction.
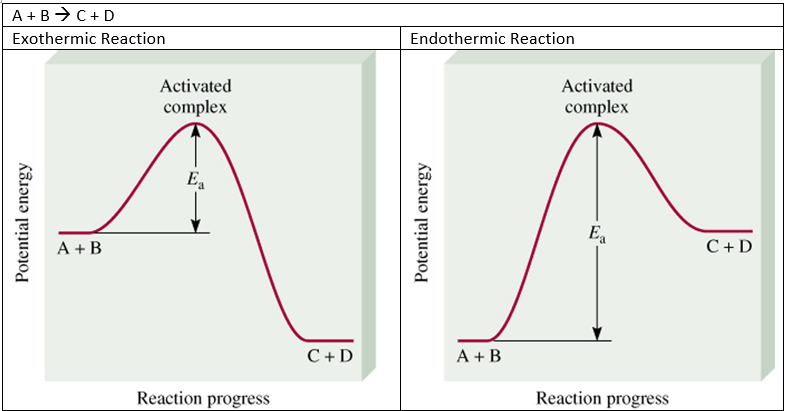
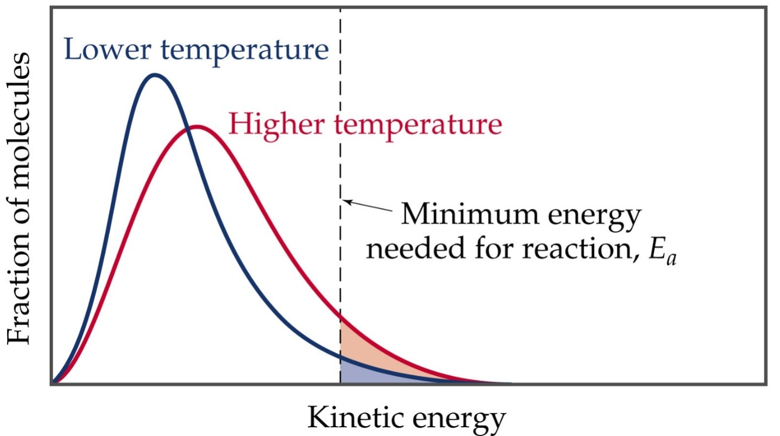
Fig 2: Temperature vs. Activation Energy.
Temperature doesn’t change the activation energy but it does provide kinetic energy to the molecules to reach the transition state faster. As the temperature increases, the curve flattens and broadens. This means that higher temperatures, a larger population of molecules obtain higher energy. If the dotted line in the figure above represents the activation energy, after that as the inversion increases, as well as the fraction of molecules that can overcome the activation energy milestone. As an outcome, the reaction rate goes up.
Figure 3: Difference between catalyzed and an uncatalyzed reaction. Ratecatalyzed>Rateuncatalysed
A catalyst is a substance that enhances the rate of a chemical reaction without itself being consumed in the reaction. It does so by decreasing the activation energy of the reaction.