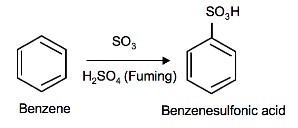
Aromatic hydrocarbon – sulphonation
In the sulphonation of benzene, a hydrogen atom attached to the carbon atom of the ring gets substituted by a sulphonic acid group. The attacking reagent is sulphur trioxide. It can be formed by the dissociation of the sulphuric acid.
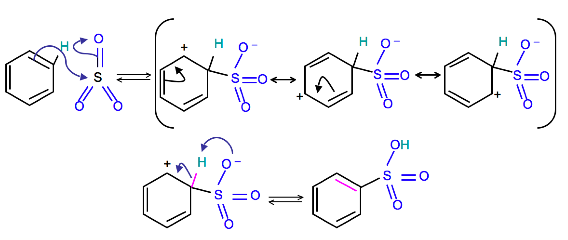
The sulphonation of benzene follows the following steps:
- The electrophile is generated as follows:Sulphur atom in sulphur trioxide has the only sextet of electrons. Therefore, the molecule is anelectron deficient in nature and acts as an electrophile. The electrophile in this reaction is sulphur trioxide. The formation of this electrophile arises in two ways depending upon the acid we use. Concentrated sulphuric acid contains traces of sulphur trioxide due to the slight dissociation of the acid.
While fuming sulphuric acid can be thought of as a solution of sulphur trioxide in sulphuric acid and is so much richer source of sulphur trioxide. Sulphur trioxide is a highly polar molecule with a pretty fair amount of positive charge on sulphur atom and this is why it is attracted to the ring of electrons and hence used as an electrophile.
- The electrophile attacks the benzene ring to form an intermediate carbocation.
- The intermediate carbocation loses a to form benzene sulphonate ion. This can take up a proton from ion to form benzene sulphonic acid.
In contrast to all other electrophilic aromatic substitution, this reaction is reversible. Aromatic sulfonation takes place in the concentrated acidic conditions and the reverse of this reaction desulphonation takes place in a dilute hot aqueous acid. The reversibility of this reaction is very important in protecting the aromatic system because of this reversibility. Due to the electron- withdrawing effects, these sulphonate protecting groups can be used to prevent electrophilic aromatic substitution.
Apart from this direct sulphonation method various method have been developed to introduce sulphonate group into an aromatic ring.
- Piria reaction: In this reaction nitrobenzene is reacted with the metal bisulphite forming an amino sulphonic acid as a result of combined nitro group reduction and sulphonation.
- Tyrer sulphonation process: In this tyrer’s reaction, the benzene vapor is led through a vessel containing 90
Now let us talk about the application of the sulphonation reaction:
- The aromatic sulphonic acids which we get from the sulphonation reaction are intermediate in the preparation of various dyes and many pharmaceuticals. Sulphonation of anilines leads to a large group of very important sulfa drugs.
- Polystyrene’s sulphonation is used to make sodium polystyrene sulphonate, a common ion exchange resin for water softening.
- The aromatic sulphonic acids which we get from the sulphonation reaction are intermediate in the preparation of various dyes and many pharmaceuticals. Sulphonation of anilines leads to a large group of very important sulfa drugs.
Reactions of aromatic sulphonic acids:
- Aromatic or aryl sulphonic acid undergo desulphonation when heated in water as shown below:
- Benzene sulphonic acid derivates converts to phenol through the phenoxides when treated with a strong base.