The replacement of a hydrogen atom in the ring of an arene by a halogen atom (F, Cl, Br, I) is called halogenations. Few types of an aromatic compound such as phenols react without the help of catalyst but the less reactive substrates such as benzene reacts in the presence of Lewis acids as a catalyst. The halogenation takes place in the presence of a Lewis acid such as ferric halide or aluminium halide which acts as halogen carrier. The chlorination and bromination occur at a temperature between 310 to 320 K. the function of Lewis acids like is to carry the halogen to aromatic hydrocarbons. Therefore, they are also called halogen carriers.
Reaction with iodine is reversible because HI formed reduces iodo derivative back to benzene. Therefore, the reaction is carried out in the presence of an oxidizing agent such as iodic acid or nitric acid
or mercuric oxide (HgO).
Fluorination is a very fast reaction and it is difficult to control the reaction. Electrophilic fluorination can be possible with or N-F reagents like selctfluor, these methods are rarely used because of the formation of isomeric mixtures and polyfluorination products. It becomes extremely challenging to separate this mixture from their nonfluorinated, polyfluorinated or isomeric counterparts.
Halogenation of aromatic compounds differs from that of halogenations of alkenes in a requirement of a catalyst as alkenes do not require a catalyst to enhance the electrophilicity of the halogen. Because of the formation of arenium ion results into the temporary loss of aromaticity, which has higher activation energy as compared to the halonium ion which is formed in the case of alkenes. We can also state that alkenes are more reactive and do not need to have Br-Br or Cl-Cl bond weakend.
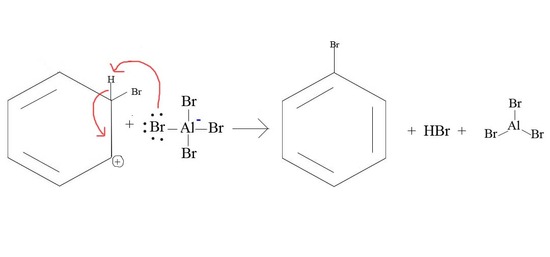
Mechanism of halogenation of benzene:
The chlorination or bromination of benzene is carried out by treating with chlorine or bromine in the presence of ferric salts i.e., . The metal catalyst being Lewis acid Fe is electron deficient polarizes the halogen molecule and thus, generates an electrophile. The mechanism is being illustrated with the help of the chlorination of benzene.
- The electrophile is generated as below:
It has been suggested that free cholronium ion may not have actual existence. The electrophile is supposed to be made available from a complex between .
Application of halogenation:
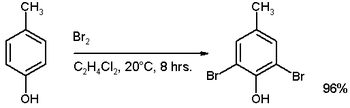
If the aromatic ring contains the strongly activating substituents such as –OH, -OR or amines, a catalyst is not necessary. For example, see the reaction of p-cresol:
However, if we use an excess of bromine then a tribromide derivative will be formed.
Halogenations of phenols are generally faster in polar solvents in a basic environment this is because of the dissociation of phenol, with phenoxide ions being more susceptible to electrophilic attack as they are more electron-rich.
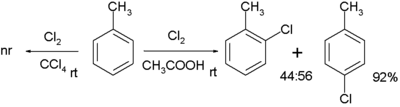
Chlorination of toluene can occur without a catalyst that requires a polar solvent such as acetic acid.
Erythrosine the well-known food dye can be synthesized by the iodination of another dye called fluoroscein. This reaction is led by sodium bicarbonate.