Aromatic hydrocarbons- Friedel craft’s alkylation and acylation
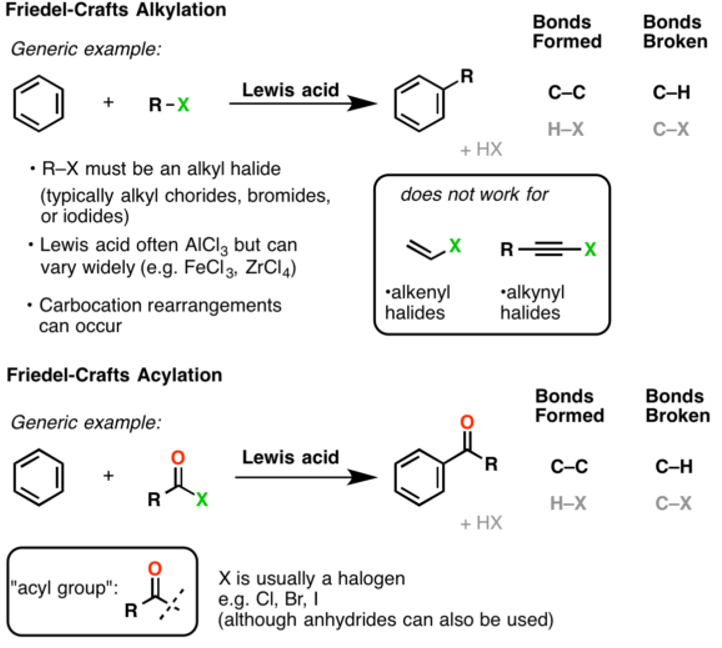
Alkylation: on the treatment with an alkyl halide in the presence of anhydrous aluminium chloride, benzene forms an alkylbenzene. The reaction is also called Friedel-crafts alkylation reaction. In this reaction, a hydrogen atom in the benzene ring is replaced by an alkyl group. For example,
Mechanism of Friedel-craft alkylation reaction:
In the alkylation of benzene, one hydrogen atom attached to the carbon atom of the ring gets replaced by the alkyl (-R) group. The attacking electrophile is an alkyl carbonium ion made available by alkyl halide molecule. The release of electrophile is facilitated by the presence of anhydrous
. The mechanism is illustrated with the help of methyl chloride,
- The electrophile (methyl carbocation) is generated as :
- The electrophile attacks the benzene ring to form an intermediate carbocation.
- The intermediate carbocation loses a proton to
ion to give an alkylated product.
When the alkylation is carried with higher alkyl halides such as n-propyl chloride, the electrophile formed in the first step is a primary carbocation being extremely unstable, it changes to a more stable secondary carbocation by the rearrangement reaction.
Thus the electrophile is ion and the product formed is 2-phenyl propane and not 1-phenyl propane. Alkylations are not limited to the alkyl halides only, they are possible with any carbocationic intermediate such as those derived from alkenes and a protic acids, enones, and epoxides. The disadvantage of the alkylation reaction is that the product is more nucleophilic than the reactant because alkyl groups are activators for the Friedel-craft reaction.
Acylation or Friedel-craft acylation reaction:
On treatment with an acyl halide or acid anhydride in the presence of anhydrous benzene forms acyl benzene. This reaction is called Friedel-craft acylation reaction.
Mechanism of Friedel-craft acylation reaction:
In the acylation of benzene, the hydrogen attached to the carbon atom of the ring gets replaced by the acyl (RCO-) group. The attacking electrophile, an acyl carbocation is supplied by acid chloride (RCOCl) in the presence of anhydrous . The mechanism is illustrated by acetyl chloride,
. Stability of acyl chloride reagent decides the viability of Friedel-crafts acylation reaction. Due to the electron-withdrawing effect of the carbonyl group, the ketone product is always less reactive than the original molecule, so multiple acylations do not occur. Hence, Acylation reaction is advantageous over alkylation reaction
- The electrophile is generated
- The electrophile attacks the benzene ring to form an intermediate a carbocation.
- The intermediate carbocation loses a proton to ion to give an acetylated product.
Scope and variation of the Friedel-craft alkylation and acylation reactions:
- Friedel-craft reactions have been used in the synthesis of several dyes such as triarylmethane and xanthenes dyes.
- The Nencki reaction is the ring acylation of phenols with acids in the presence of zinc chloride.
- In the Nenitzescu synthesis of ketones involves acylation of cyclohexene with acetyl chloride to methylcyclohexenylketone.
- The acylation of cyclohexene with acetyl chloride to metgycyclohexenylketone is called the Darzens-Ninitzescu synthesis of ketones.
- In the reaction called Zincke-suhl reaction p-cresol is alkylated to a cyclohexadienone with tetrachloromethane.