Aromatic hydrocarbons- The Directive influence of the various functional group in mono-substituted benzene
As we have learned, all the six hydrogen atoms of benzene rings are equivalent. Therefore, replacement of any one of these six hydrogen atoms by any substitution always forms a single mono substituted product. However, when mono-substituted benzene is subjected to further substitution, the group present on the benzene ring affects the incoming attacking groups.
The ability of a group already present in the benzene ring to direct the incoming group to a particular position is called the directive influence of groups.
A substituent already present on the ring has two effects:
- Orientation effect: substituents affect the orientation of the reaction. The three possible disubstituted products i.e., ortho, para, and, meta are not formed in equal amounts. The nature of the substituents already present on the benzene ring determines the position of the second substitution. In this way, it has been found that every group can be put into one of the two classes: ortho-para directing and meta –directing group.
- Reactivity: the substituents affect the reactivity of the aromatic rings. Some substituents activate the ring and therefore make it more reactive than benzene. On the other hand, some groups deactivate the ring and make it less reactive than benzene. For example, in aromatic nitration, the –OH group makes the ring more reactive than benzene, whereas a nitro group makes the ring less reactive.
The substituents can be classified into three groups:
- Ortho and para-directing activators: These groups release electrons and activate the benzene ring. These groups direct the incoming groups to ortho and para positions. The common examples are:
etc.
Let us discuss the influence of –OH group. The resonance structure of phenol shows that the overall electron density on the benzene ring increases in comparison to benzene. Therefore, it is an activating group. The typical reactions of aromatic hydrocarbons are electrophilic substitution reactions in which electrophile attacks the benzene ring.
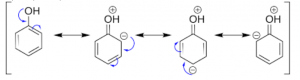
- Meta directing deactivating groups: These groups withdraw electrons from the benzene ring and deactivate it. These groups direct the incoming group to meta positions. The common example is:
Let us discuss the influence of the nitro group. From the resonance structures, we can say that the overall density on the benzene ring decreases and therefore, this group is the deactivating group. Moreover, electron density on the ortho and para position is less than on the meta position. Therefore, the two meta positions are rich in electron density, thus resulting in meta substitution.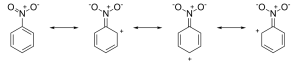
- Ortho and para directing deactivating groups: These groups withdraw electrons from the benzene ring and deactivate it. However, these direct the incoming group to ortho and para positions. The common examples are:
The halogens have a unique character in being weakly deactivating but ortho and para directing.The halogens are highly deactivating because of their strong negative inductive effect. Therefore, the overall electron density on the benzene ring decreases. However, due to resonance, the electric density on ortho and para position is more and thus these are ortho and para directing.