Electrical Properties of Solids
Classification of Solids on the Basis of Electrical Conductivity:
Solids display a deviating range of electrical conductivities, expanding of magnitude right from 10 to the power of (-20) to 10 to the power of (7) ohm-1 m–1.
Solids can be divided into 3 types on their conductivities’ basis.
1. Conductors:
- The conductivity ranges between 10 to the power of 4 to 10 to the power of 7 ohm-1 m–1
- Metals are having conductivities in the range of10 to the power of 7 ohm-1 m–1.
- Eg: Aluminium, Copper, Gold, Silver, All metals
2. Insulators
- Insulators are the solids having very very small conductivity range between 10 to the power of (-20) to 10 to the power of (-10) ohm-1 m–1.
- Eg: Wood, plastic, rubber, glass, mica
3. Semiconductors
- The metal conductor has the ability to conduct electricity via the electrons’ movement. In both state, that is, in molten as well as in solid, metal has the property of conducting electricity.
- Metals’ conductivity depends on the availability of valence electron’s number per atom
Magnetic Properties of Solids
We suppose everyone is aware of the magnets, right? Well, apparently you may also be knowing about the metals which are magnetic in nature. But the question is do you know what the magnetic property of solids are due to their atomic structure? Yeah, it does! So, let us now focus our knowledge on various kinds of magnetic solids.
Solids’ Magnetic Properties
Solids’ magnetic properties are the consequences of ions’ or atoms’ magnetic property. To be more specific, the magnetization and magnetism of a solid in an atom are dependent on electrons’ movement. Thus, we can say that each of the atoms’ electron has the behavior of acting like a magnet, which lends (loan) an entire solid with its magnetic properties.
So, the magnetic behavior of electrons of the atom is because of the movement patterns. They arecomprised of two movement types, in particular,
- The electrons move around or revolve around atoms’ nucleus.
- Also, electron spin on its own axis, i.e. when it spins in the opposite sides, they are marked with the signs – and +
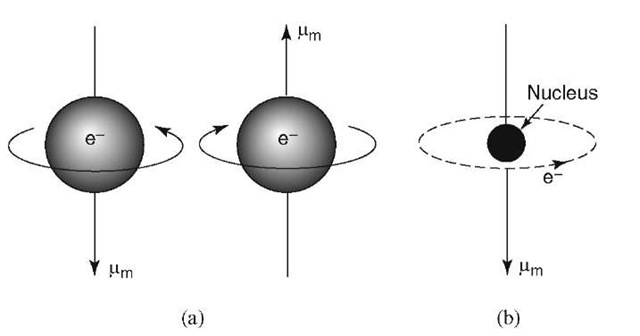
These respective two electrons’ movements provide the substance and the atom with their magnetic strength.
These continuous movements result in an electric field which surrounds the electrons, which is approximately like a current loop, lending its magnetic property to it. On magnetic properties’ basis, solids are classified into five categories.
Paramagnetic
The properties of paramagnetic substances can be understood as:
- The magnetism of these substances are weak in nature in the magnetic field which acts externally.
- The direction is exactly the same as magnetic’s field direction
- Electron’s alignment is disrupted and hence the magnetic properties of the substance are lost
- In nutshell, paramagnetic substances don’t exist as permanent magnets
- Eg: Mg, Li
Diamagnetic
- In this, the magnetism of substances takes place in the magnetic field which is external, just as in the case of paramagnetism
- The solids of diamagnetic substances are repelling in that external magnetic field and therefore, consist of a repulsive force
- The last shell electrons of this category are paired and thus, there are no valence electrons
- The magnetic property is therefore zero
- They are used as insulators
- Eg: Cu, Au, Hg, H, alcohol
Ferromagnetic
- These solids are having a strong magnetism
- They are placed in a magnetic field which is external
- These solid types have the property of magnetizing permanently
- They have strong forces of attraction
- Eg: Fe, Ni, Co