Surface Chemistry: Catalysis
According to the collision theory, particles need to have at least a minimum amount of activation energy to initiate the reaction. Catalysis is a process in which a catalyst is used to reduce the activation energy of a reaction by changing the path of the reactants. It is the manifestation, study, and use of catalysts and catalytic processes. A Catalyst is a substance that affects the rate of a chemical reaction without reduced its own constituents. Catalyst affects only the rate of the reaction which is corresponding to its kinetics. It does not alter the thermodynamics or the equilibrium composition of the reaction. It is mainly involved with lowering the activation energy for both forward and reverses reactions. Likewise, catalytic activity is dependent upon the adsorption of a reactant, which is determined by the surface solid state of a catalyst. Hence, adsorption plays an important role in catalysis of the reactants.
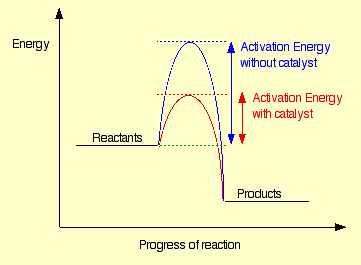
Figure 1: Effect of catalyst on a reaction.A greater proportion of particles will have more energies than Easo more will react
If а catalyst accelerates the reaction speed, it is defined as a positive catalyst and the phenomenon is called positive catalysis. Alternatively, if а catalystretards the reaction speed, it is called а negative catalyst and the phenomenon is called negative catalysis.
Catalysis is also dependent on the proximity and orientation of the reactants. Reactions are not catalyzed over the entire surface of the bed reactor but only at certain specific active sites or centers that result from unsaturated atoms in the surface. An active site is a point on the surface that can allow the formation of strong chemical bonds with an adsorbed atom or molecule. Also, when the local concentration of the molecules increases, their interaction increases. A catalyst allows reactants to stay in the optimum position to increase the probability of collision of molecules in the correct orientation.
Electrostatic catalysis causes local dielectric constant to decrease to enhance electrostatic interactions on the active site. It also prevents the reaction of unwanted by-products. Acid-Bases catalysis e.g., hydrolysis of ester/ peptide bonds, phosphate group reactions, addition to carbonyl groups, etc. allows the specific catalytic cycle of proton exchange. Covalent catalysis allows the transient formation of a catalyst-substrate covalent bond to increase the rate of the reaction. Metal ion catalysis is often used in more than one way. Metal ions allow binding substrates in the proper orientation, mediation of oxidation-reduction reactions, electrostatic stabilization or shielding negative charges that would otherwise repel the attack from an electrophile (electrostatic catalysis) and simply stabilization of the catalytically active form of the catalyst.
Catalysis of gaseous reactions can increase the rate in several pathways:
- Proximity: -species adsorbed onto the surface and is more likely to undergo a collision
- Orientation: -species held in a favourable position for reaction to occur
- Transition state: -adsorption onto the surface facilitates bonds to break and fragments react quicker
- Surface area to volume ratio: – when two reactants are adsorbed alongside each other, they induce a greater concentration
There are two types of catalysts:
Homogeneous: Catalyst and Reactant are in the same phase
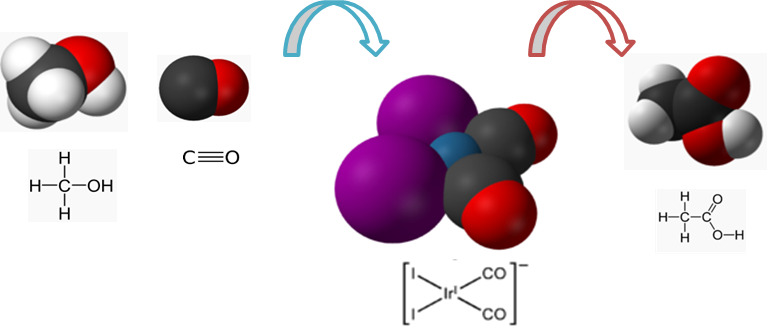
Fig 2: Catalysis in a homogeneous catalysis of organometallic compounds.
Heterogeneous: Catalyst and Reactant are in the different phases
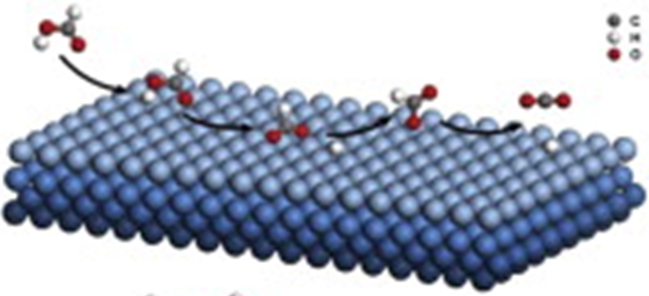
Fig 3: Metal surface catalysis