Surface Chemistry: Homogenous and heterogeneous catalysis
Activity and Selectivity
Activity is the ability of the catalyst to accelerate a chemical reaction. The degree can be as high as 100 times in certain reactions. A catalytic cycle processes in which the reactant and catalyst undergo several transformations before making the products. A catalyst should regenerate after every cycle. The higher no. of times a catalyst regenerates, the higher is its activity. The number of times that a catalyst follows through this cycle of converting the substrate molecule to product molecules is defined as its turnover number.
Selectivity is the ability of catalysts to direct reaction to yield particular products and exclude others. Catalysts are highly selective in nature. For example, consider two chemically variant functionalities are present in the same molecule. When a selective reaction of one functional group occurs while avoiding the other, it is known as chemoselectivity. When the functional group can attach to multiple sites, then the selectivity that determines the site of attachment is known as regioselectivity. For a substrate containing a stereogeniccentre, the catalyst can direct the addition of atoms to give two diastereomers. The selectivity for either diastereomer is called diastereoselectivity
Complete oxidation example (nonselective)
The adsorption of C2H4 and O2 onto the catalyst Pt provides a chemical shortcut → reaction at lower temperatures
Partial oxidation example (selective)
The catalyst manipulates the selectivity by preferentially lowering the activation energy for one step in the reaction sequence while increasing the rate at which this step proceeds.
e.g., V2O5:The reaction path leading to the formation of the aldehyde product is favoured because it has a lower activation energy than the complete combustion to CO2 and H2O.
Pt: The opposite is true.
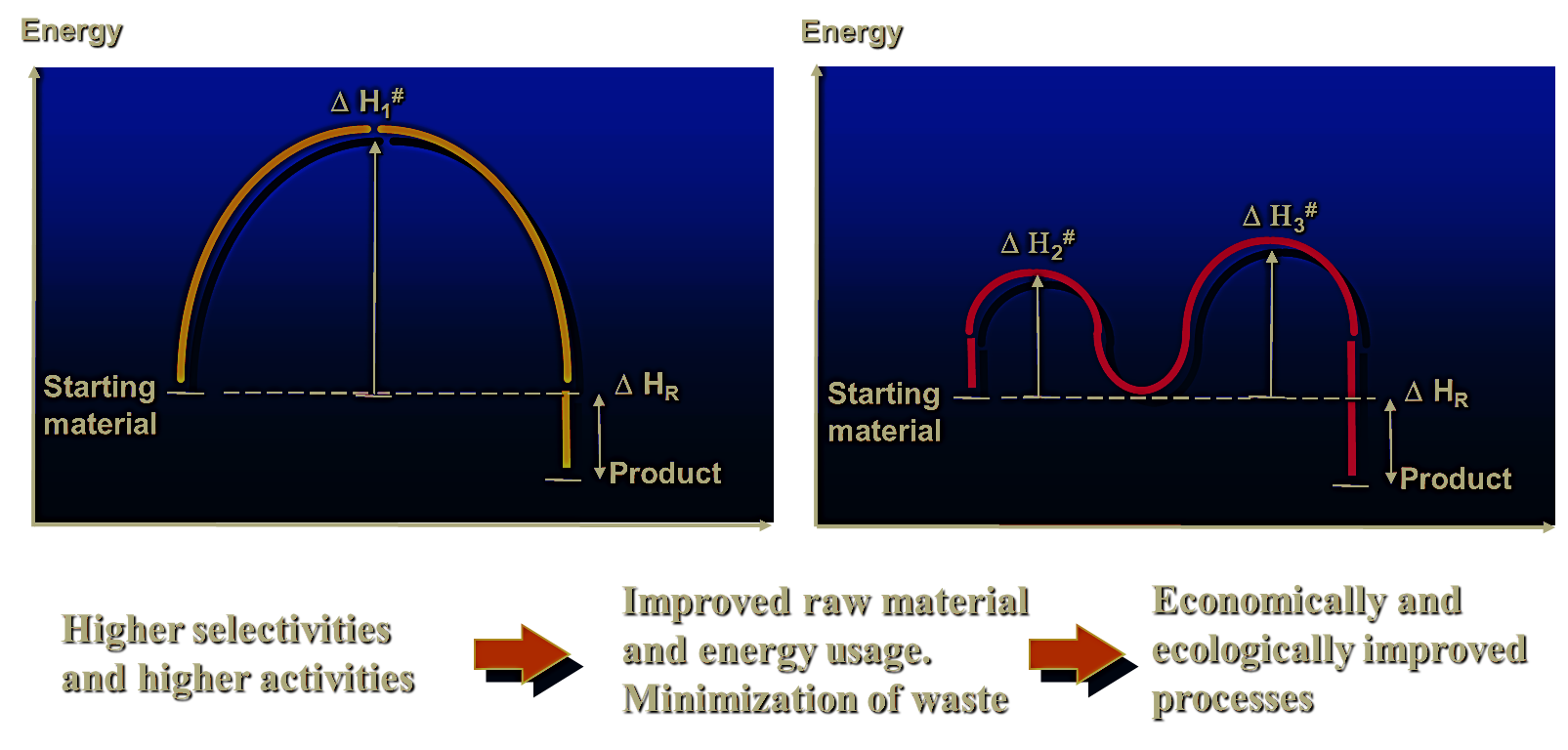
Fig 1 : Catalytic activity
Heterogeneous catalysis
Catalysts are present in a different phase as oppose to the reactants. e.g. a solid catalyst in a gaseous reaction. It takes place at active sites on the surface of solid gas are adsorbed onto the surface they form weak bonds with metal atoms.
These reactions can be explained based on intermediate compound formation. According to this theory, the catalyst combines with one of the reactants to form an intermediate. The intermediate compound being unstable combines with the other reactant to form the product. For example, the combination of SO2 and O2 to form SO3 is a slow process. However, in the presence of NO (catalyst), the reaction becomes fast.
Fig 2: Step 1: Adsorption, Step 2 and 3: Reaction, Step 4: Desorption
Step 1: Diffusion of the reactants at the surface of the catalyst takes place. Incoming species lands on an active site to bond with the catalyst. Bonding electrons in the molecules are used to weaken them and make a subsequent reaction easier.
Step 2: Adsorption of the molecules of the reactants at the active sites is the second step. Adsorbed gases may be held on the surface in just the right orientation for a reaction to occur.This increases the probability of favourable collisions taking place.
Step 3: Occurrence of the chemical reactions on the surface of the catalyst.
Step 4: Desorption of products molecules from the surface. Diffusion of products away from the surface of the catalyst follows after the reaction. There is a re-arrangement of electrons and the products are then released from the active sites
Selectivity is low in homogeneous catalysis. It is a consequence of more difficult fine-tuning of the steric and electronic properties of the catalyst and of the more difficult way of finding out the reaction mechanism.
Homogeneous catalysis
In homogeneous catalysis, the catalyst and reactants are in the same phase. The reaction proceeds through an intermediate species of lower energy. There is usually more than one reaction step. Transition metal ions are often involved in this type which allows oxidation state changes.
Consider the following reaction:
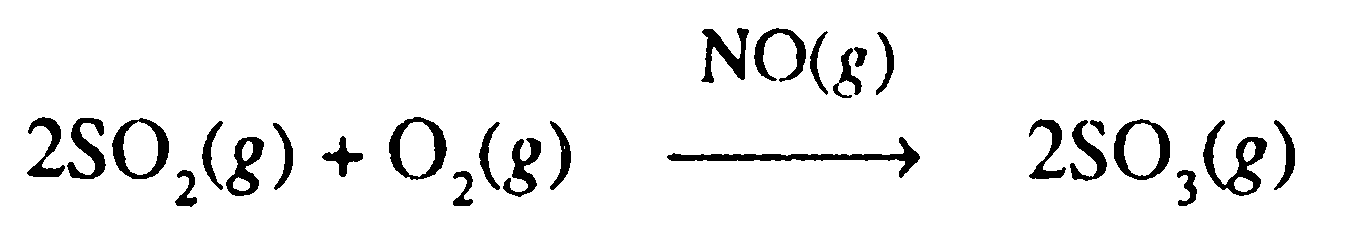
The intermediate steps are:
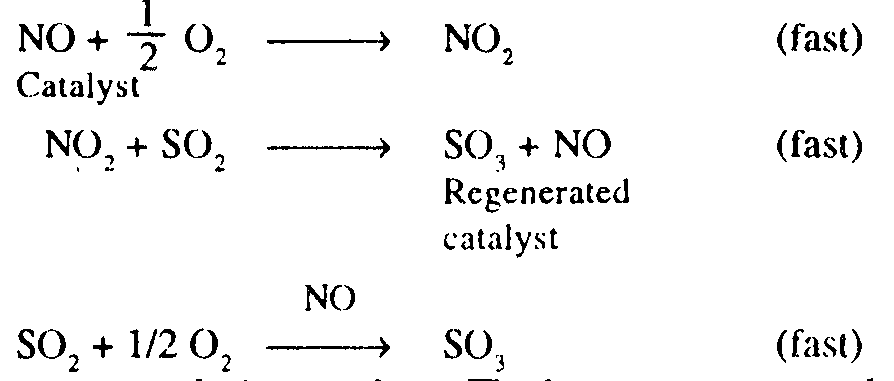
Selectivity is high in homogeneous catalysis. It is a consequence of the easier fine-tuning of the steric and electronic properties of the catalyst and of the easier way of finding out the reaction mechanism.
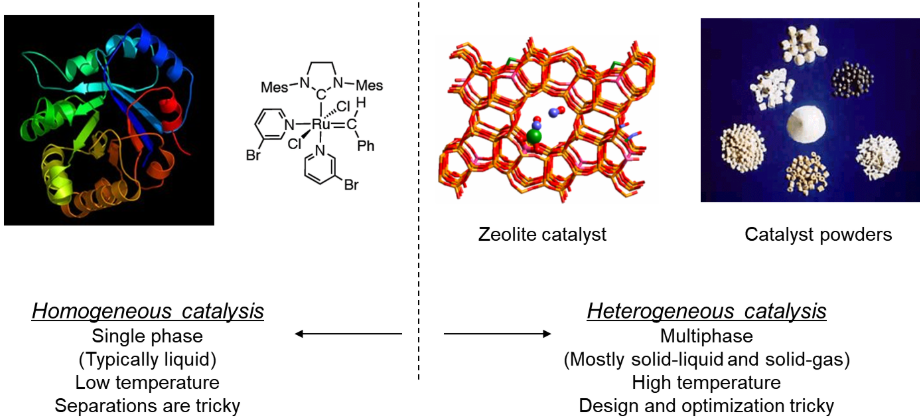
Fig 3: Homogeneous catalyst vs. heterogeneous catalyst