Surface Chemistry: Tyndall effect
A beam of sunlight is not visible to the naked eye unless the light passes through particles of water (mist) or dust in the air. These particles are responsible to scatter the sunlight and show its direction of penetration. Similarly, the light becomes is visible as it passes through a colloid. Opalescence in colloids is a result of light scattering by particles. Tyndall effect is quite strong in lyophobic colloids while in lyophilic colloids it is quite weak.
Tyndall observed that scattered beam to be polarized and intensity of the same to be dependent on the position of an observer, nature of system and wavelength of light used. The scattering of visible light by colloidal particles is called the Tyndall effect or Tyndall scattering. Suspensions also exhibit the Tyndall effect. The particles in solutions are less than 1 nm which makes them smaller than the wavelength of visible light.
Tyndall effect is like Rayleigh scattering, where the intensity of the scattered light depends on the fourth power of the frequency. This means that blue light is scattered much more strongly than red light. For instance, the blue colour is often seen in the smoke emitted by motorcycles, which are two-stroke machines where the burnt engine oil produces particles.
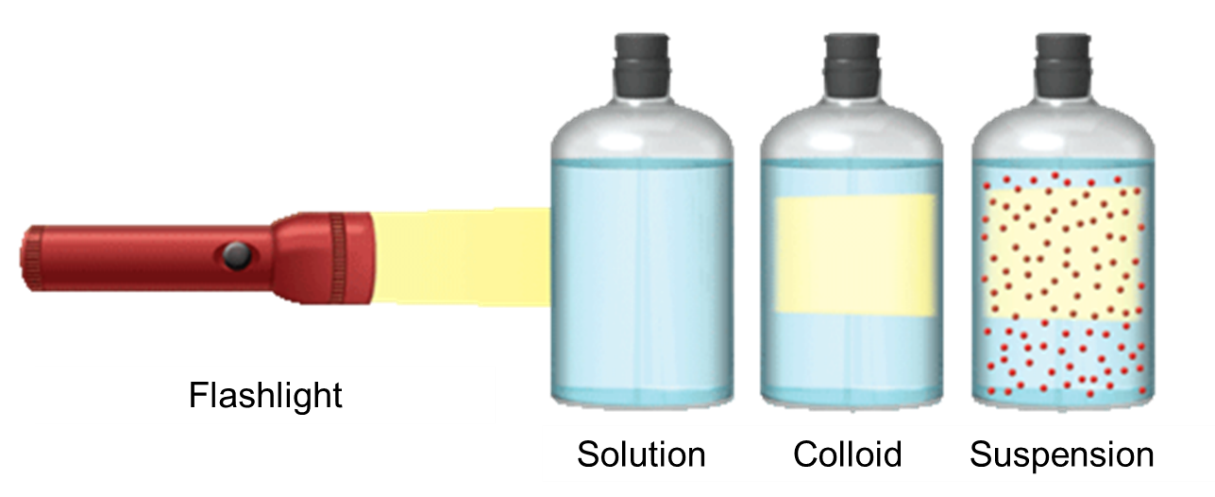
Figure 1: When a beam of light falls at right angles to the line of view through a solution, the solution appears to be luminescent and due to the scattering of light the path becomes visible.The light beam becomes visible as it passes through a colloid or suspension because particles scatter light.This distinguishes a colloid from a solution and the scattered light is mostly blue.
Light scattering measurements are of great value for estimating particle size and shape and number of particles per unit weight or volume. Turbidity in a liquid is because of the presence of finely divided suspended particles. If a beam of light passes through a turbid sample, scattering reduces its intensity, and the quantity of light scattered relates to the concentration and size distribution of the particles. Turbidimetry is the measurement of the intensity of light transmitted through the sample where the unscattered light is measured using a spectrophotometer. The intensity of scattered light is can also be measured by a nephelometer.
Table 1: How can you tell the difference between a solution, a colloid and a suspension? If the mixture does not filter particles, considerthe Tyndall effect.
| Type of mixture | Approx. size of the solute particle | Do particles settle? | Will filtering separate particles? | Do particles scatter light? |
| Solutions | 0.01 to 1.0 nm | no | no | No |
| Colloids | 1.0 to 1000 nm | No | Only with special equipment | Yes, if translucent |
| Suspensions | >1000 nm | yes | yes | Yes, if translucent |
Examples of the Tyndall Effect:
Milk is a colloid containing globules of fat and protein. When a beam of light is passed on a glass of milk, the light is scattered.
When a torch or car’s headlights are switched on in foggy weather, the path of the light becomes visible. In this case, the water droplets in the fog are responsible for the light scattering.
Opalescent glass has a bluish appearance when it is viewed sideways. However, orange-coloured light is visible when light is directed through the glass.