Ozone layer depletion is characterised by the large, seasonal, decrease in stratospheric ozone over Earth’s polar regions and a slow decline of four per cent in the total volume of Ozone in Earth’s ozone layer since the ’70s. A balance between the photochemical production and recombination determines the overall amount of the Ozone in the stratosphere.

The manufacture of Ozone-depleting substances (halocarbon refrigerants, chlorofluorocarbons form blowing agents and solvents) is the leading cause of ozone depletion and the ozone hole. Through photo-dissociation, halogen atoms are released from molecules formed through turbulent mixing and transportation of these compounds from the surface to the stratosphere. These atoms catalysethe breakdown of the Ozone into oxygen. Marked decreases in column ozone have been observed although the full extent of the damage caused by CFCs to the ozone layer is unknown. Efforts to replace CFCs by the less damaging hydro-chloro-fluoro-carbons (HCFCs) are ongoing, despite concerns regarding them. A decrease in bromine-containing chemicals has also significantly reduced the effects caused by the ozone layer. Ozone-depleting chemicals are also greenhouse gases. With the increases in chemical concentration have produced radiative forcing, which is about 14% of the total radiative forcing from increases in the levels of greenhouse gases.
Carbon dioxide, radiative forcing that produces global warming also cools the stratosphere. This cooling provides a relative increase in ozone depletion in polar areas and the frequency of ozone
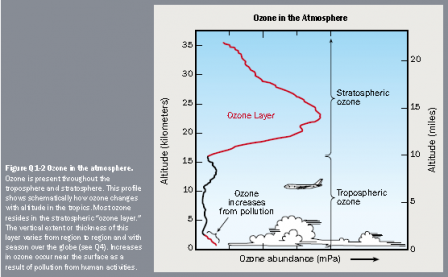
holes. The depletion of Ozone represents a radiative forcing of the climate system. The stratosphere is made to absorb less solar radiation; thus the troposphere is warmed while the stratosphere is cooled; as a result, the colder stratosphere emits less longwave radiation downward and in return, cooling the troposphere.
The effects of this depletion have been felt and experienced throughout the world and have generated concerns over increased cancer risks and other negative effects. This is because the Ozone is responsible for preventing most harmful Ultraviolet light and different UVB wavelengths from passing through our atmosphere. The increased levels of skin cancer, cataracts and sunburns have been attributed to the thinning Ozone and increase in human activity and their energy-intensive lifestyles.
In recent times, governments and various non-profit organisations have banned the production of halons, CFCs and other Ozone-depleting chemicals. Adoption of the Montreal Protocol has led to the reductions in the emissions of CFCs leading to a decline of the most significant of these compounds in the atmosphere