Surface Chemistry: Types of emulsions
An emulsion is a colloidal dispersion of liquid droplets (dispersed phase) of a certain size within a second immiscible liquid (continuous phase).An emulsifying agent is crucial for the formation of an emulsion and for maintaining the emulsion’s stability to increase its shelf life.Emulsions are usually made by vigorously mixing the two constituents together along with the addition of an emulsifying agent. The phase in which emulsifier is more soluble forms the outer layer. Emulsions are lyophobic colloids.
Emulsions are thermodynamically unstable and coarsely dispersed systems of two immiscible liquids in which the liquid acts as the dispersed phase as well as the dispersion medium. They are obtained by mixing oil with water. Since the two do not mix well, the emulsion is generally unstable and is stabilized by adding an emulsifier or emulsifying agent (gum, soap, glass powder, etc).Emulsions are unstable because the globules of the dispersed liquid tend to coalesce to form large globules until all of the dispersed globules have coalesced.
Consider the figure below where one end of a large soap or detergent molecule is polar and is attracted to water molecules.The other end of the soap or detergent molecule is nonpolar and is soluble in PFC or some other kind of oil or grease.Soaps and other emulsifying agents thus allow the formation of colloidal dispersions between liquids that do not ordinarily mix.
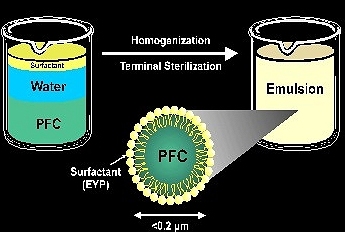
Fig : Surfactant molecule in an emulsion.
Type of an emulsion is associated with the phase in which emulsifier is placed.Emulsifying agents that are preferentially oil-soluble are used in W/O emulsions while polar and water-soluble emulsifying agents are used in O/W emulsions.Likewise, if the oil phase is more polar, then the emulsifier is more hydrophilic. More non-polar the oil phase, then more lipophilic the emulsifier should be.
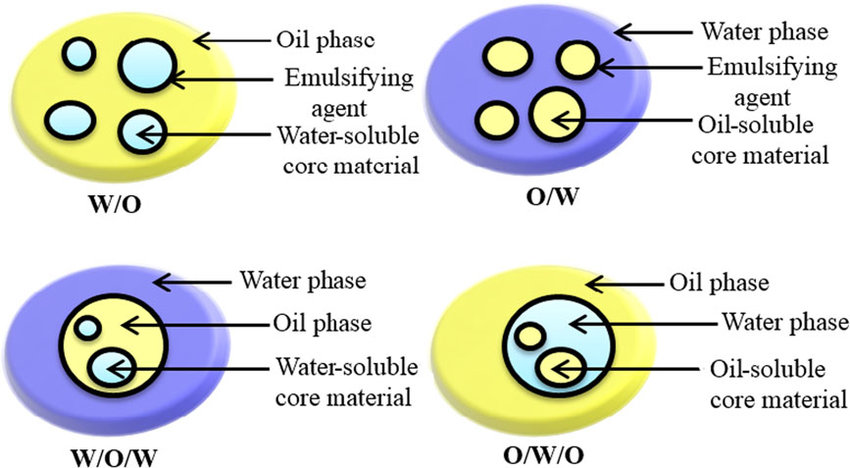
Fig : Types of emulsions
Oil in water emulsion is one where oil is the dispersed phase and water is the dispersion medium, e.g. milk.In this case, emulsifying agents are more soluble in water than in oil. O/W emulsions give +ve conductivity test because water is a good conductor of electricity.
Water in oil emulsion is one where water is the dispersed phase and oil is the dispersed medium, e.g. butter, cream.In this case, emulsifying agents are more soluble in oil than in water. W/O emulsions do not give a positive conductivity test as oil being the external phase does not conduct electricity.
Water in Oil in water (W/O/W)is one where water in Oil emulsion is dispersed in water creating multiple emulsions. O/W/O emulsion is the opposite of the later. These types of emulsions help in delayed release of certain small compounds used in the pharmaceutical industry.
Amphiphilic surfactants can be catagorised by the hydrophilic-lipophilic balance (HLB) which is a relative ratio of polar and non-polar groups in particular surfactant.
- HLB ca. 3.5 to 8: Water-in-Oil Emulsifiers
- HLB ca. 1 to 3.5: Antifoams
- HLB ca. 7 to 9: Wetting and spreading agents
- HLB ca. 8 to 16: Oil-in-Water Emulsifiers
- HLB ca. 13 to 16: Detergents
- HLB ca. 15 to 40: Solubilizers
| Type | Disperse phase | Continuous phase |
| Emulsion: o/w | Oil | Water |
| Emulsion: w/o | Water | Oil |
| Suspension | Solid | Water or oil |
| Aerosol | Solid or liquid | air |
| Others | Multiple emulsion: w/o/w, o/w/o | |