General Principles and Processes of Isolation of Elements:
Aluminium Extraction
Al is the most abundant metal in the Earth’s crust. It is a very important metal to be extracted from earth crust because:
- It is malleable
- It conducts heat and electricity
- Has lower density than iron yet stronger than steel
- Resistant to corrosion due to the formation of a protective layer of aluminium oxide.
Aluminium is a reactive metal that is naturally found as an ore called bauxite combined that contains about 30% aluminium oxide (Al2O3). Heating and Electrolysis is used to break down the Al2O3into aluminium and oxygen. As the aluminium loses oxygen, reduction takes place.
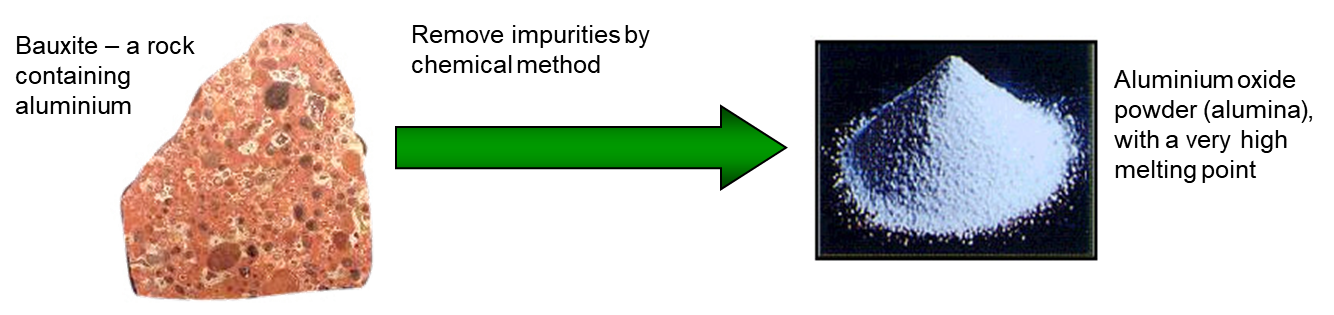
Figure 1: Bauxite contains alumina (Al2O3 aluminium oxide) plus impurities such as iron oxide – it is purified before use.
The major ore of Al is bauxite, which contains 30% of Al2O3. The Bayer process is a name given to the pretreatment of bauxite where it is boiled with NaOH. Bauxite is digested or leached with a solution of sodium hydroxide NaOH, at high temperature, under high pressure. Depending on the bauxite grade the temperature varies between 145 -260 °C
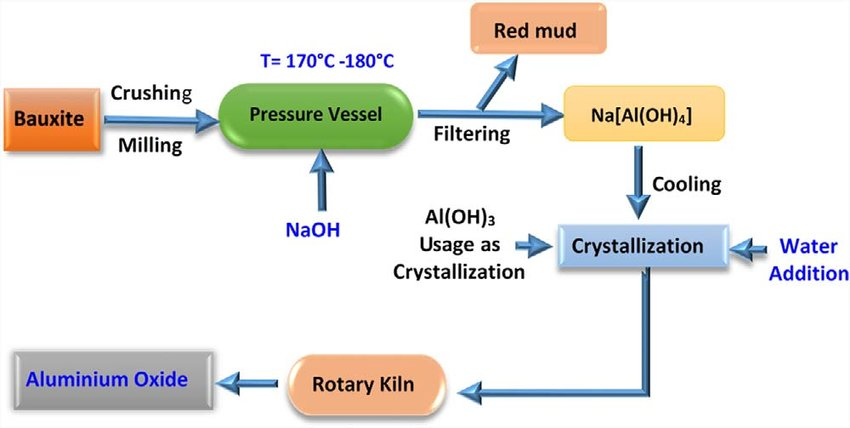
Fig 2: The Bayer Proces.
Aluminium ore (bauxite) is more reactive than carbon and has a very high melting point (2050 °C). Likewise, the extraction of aluminium is an expensive process because of the high cost of electricity. Extraction factories are mostly located near a cheap source of electricity e.g. hydroelectric power.
In the reactivity series, Aluminium is placed above carbon, so it cannot be extracted from its ores in the same way as other metals such as Iron. Electrolysis of molten aluminium ore (alumina) or smelting must be used. Hall-Heroult electrolytic process is the smelting method used to convert Al2O3 into the free metal, Al.
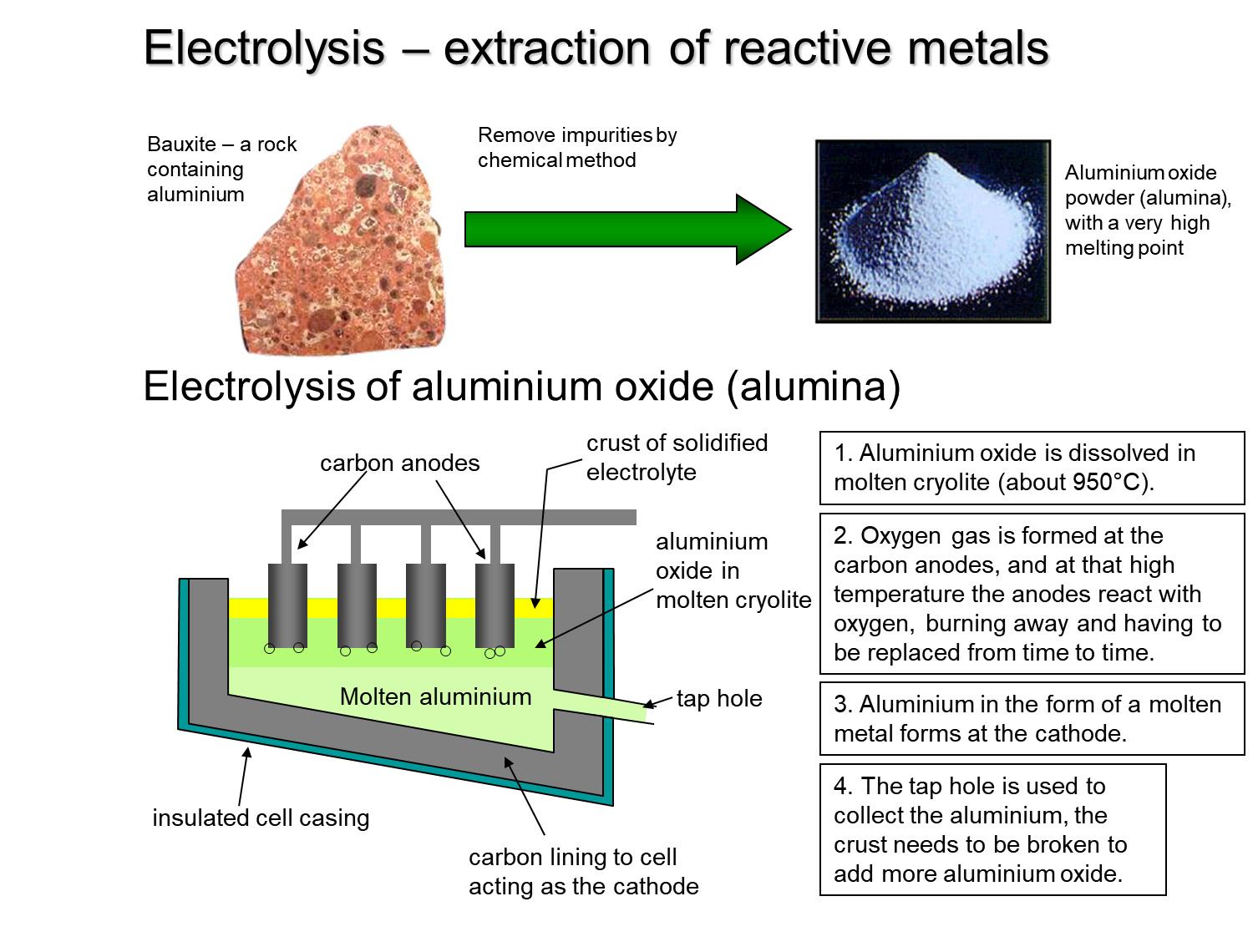
Fig :Electrolysis of aluminium oxide (alumina)
Aluminium oxide has a very high melting point. Cryolite,Na3AlF6is added to lower the melting point and save some energy. Aluminium oxide dissolves in molten cryolite and the melting point changes to about 950°C.A mixture of alumina and cryolite forms an electrolyte that is placed in an iron/steel vat lined with graphite. The vat serves as the cathode. Carbon anode is inserted into the electrolyte from the top. Oxygen gas is formed at the carbon anodes, and at that high temperature, the anodes react with oxygen, burning away and having to be replaced from time to time. Aluminium is collected as a molten metal at the cathode. A tap hole is used to collect this molten aluminium. Therefore, the crust needs to be broken to add more aluminium oxide in the electrolytic cell.
POSITIVE IONS MOVE TO THE NEGATIVE ELECTRODE (carbon lined steel cathode) = Reduction
Al3+ + 3e–Al
Balanced equation: 2Al3+ + 6e– 2Al
NEGATIVE IONS MOVE TO THE POSITIVE ELECTRODE( carbon anode) = Oxidation
O2O + 2e–
Balanced equation: 3O2-1.5O2 + 6e–
Overall reaction: 4Al3+ +6O2- 4Al + 3O2
The carbon anode reacts with oxygen to produce carbon dioxide. This means that the anodes need to replaced at regular intervals, thus adding to the cost of the extraction process.