Electrolysis Process
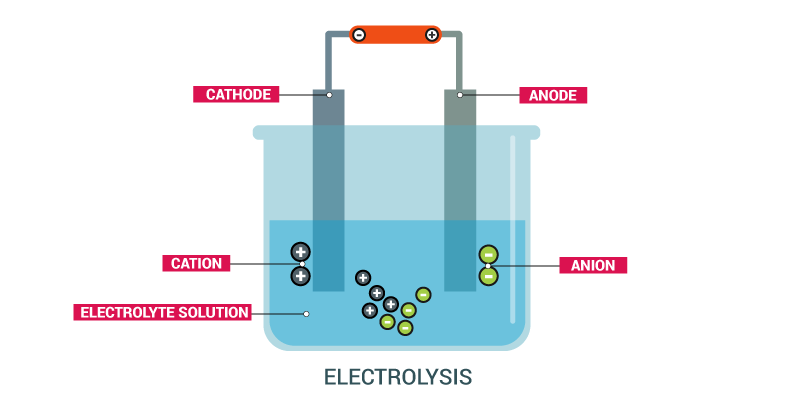
In simple words, the electrolysis process is the decomposition of the given element under the impact of the electric current.
Or,
Electrolysis refers to a method which uses a DC (direct current or direct electric current) to operate in other respects, the chemical non-spontaneous reactions.
As per the financial perspective, electrolysis plays an important role in elements’ separation from the naturally occurring sources like ores with the use of an electrolyte. In this, the voltage which is required for the electrolysis process to take place is known as the decomposition potential.
The relationship between the amount of electric charge passing through the electrolyte and the quantity of substance accumulated at electrodes was introduced in 1834 by Faraday, in the “law of electrolysis” form.
Faraday’s First Law
As per the law, substance’s mass liberated or deposited at any electrode is proportional directly to the quantity of electric charge passed through. That is, w a q
Where,
w = substance’s mass liberated or deposited
q = quantity of electric charge passed
This proportion can be assembled into equality by:
w = zq
Where,
z = proportionality constant known as the electrochemical equivalent
It’s called the substance’s mass in gms liberated or deposited by letting pass one-coulomb charge.
Faraday’s Second Law
Faraday’s second law describes that substance’s mass liberated or deposited at any electrode by passing a specific amount of electric charge is proportional in a direct manner to its chemically equivalent weight.
That is w a E
Where,
w = substance’s mass in gms
E = chemical equivalent weight, calculated in grams as per equivalent = ![]()
w = E ![]() moles of electrons
moles of electrons
1 mole of electrons is having charge:
= 1.6 ![]() 10–19
10–19 ![]() 6.023 x 1023 ≈ 96500 C
6.023 x 1023 ≈ 96500 C
This charge is known as the 1 Faraday.
![]()
So, if one Faraday charge is passed, it means that we are letting to pass electron of one mole and by the passage of one Faraday charge of 1 gram, substance’s equivalent weight will be liberated or deposited.
On combining, the first law and the second one, we get
![]()
The summarization of Faraday’s laws can be done as:
M = QM / Fz
where:
- m = substance’s mass liberated at the electrode in gms
- Q = total electric charge which passes through a substance in coulombs (C)
- F = Faraday constant whose value is 96485.33289(59) C mol−1
- M = substance’s molar mass in gms/mol
- z = ions’ valence number of a substance where the electrons are transferred per ion
Points to Remember,
M/z is identical to substance’s equivalent weight, which is changed.
In the Faraday’s first law:
The constants are F, M, and z, as in case, larger is the O value, larger the ‘m’ will be.
In Faraday’s second law:
F, Q, and z are constants, in case, the larger is the M/z value (value of equivalent weight), the larger is the m.