p-Block Elements: Group 15
Occurrence
Like the group 14 elements, the lightest member of group 15 is nitrogen which is found in nature as the free element. The heaviest elements are well known from centuries because they can be easily isolated from their ores.
Molecular nitrogen comprises 78-80
Phosphorous is mainly obtained from phosphate rock and remains of fossil life forms. Phosphorus happens to be the 11th most abundant mineral in the earth’s crust. Apatites such as Ca(PO4)3 and Ca5(PO4)3OH contain phosphorus which is extracted using sand and coal.
Ca(PO4)3F (s) + H2SO4(l) 3H3PO4 (l) + 5CaSO4 (s) + HF (aq)
At 1500 °C
Ca3(PO4)2 + 6SiO2 +10C 6CaSiO3 +10CO +P4
Unfortunately, elemental phosphorus is volatile and highly toxic. It is absorbed by the calcium present in the teeth and it also destroys bone in the jaw. This leads to a painful called “phossy jaw,” which had no cure for many years and was accepted as an occupational hazard of working in the match industry. Black phosphorus is the most stable form which is produced when P is heated under high pressure. Phosphoproteins are present in bone marrow, milk and eggs. Other phosphates are present in fertilizers.
Arsenic, antimony and bismuth are found mainly as sulphide minerals in the earth’s crust.At industrial scale, arsenic is extracted by heating appropriate minerals in the absence of air. These minerals include Realgar (As4S4), Orpiment (As2S3), Arsenolite (As2O3), arsenopyrite (FeAsS) and Ioallingite (FeAs2). Orpiment has been used by physicians and professional assassins as a poisonous substance.
For example,
FeAsS (700°C) FeS + As(g) + As (s)
Antimony is found in nature as a number of minerals such as Stibnite (Sb2S3) and Ullmannite (NiSbS). Some other ores can be treated under reducible conditions to form Sb2S3. The sulphide is removed to obtain antimony and scrap iron.
Sb2S3 +3Fe 2Sb + 3FeS
Other ores can be heated to produce Sb2O3 as in another process. The oxide can be reduced to a free element by using charcoal in the presence of sodium sulphate so that elemental antimony is formed in the mixture.
Sb2O3 +3C 4Sb + 3CO2
Bismuth is found as minerals called bismite (Bi2O3), bismuthinite (Bi2S3), and bismutite [(BiO)2CO3]. However, it is mainly produced as a byproduct of copper, lead, tin, silver, gold and zinc plants. The final step involved a reduction of the oxide of bismuth and other metal by using charcoal reducting in heat.
This is the reason why for centuries chemists were confused about its occurrence. They mistook it for other elements due to its slightly pinkish white lustre. Bismuth is widely used in printing because it is one of the few elements whose solid state is less dense than the liquid. Consequently, its alloys expand as they cool, filling a mold completely and producing crisp, clear letters for typesetting.
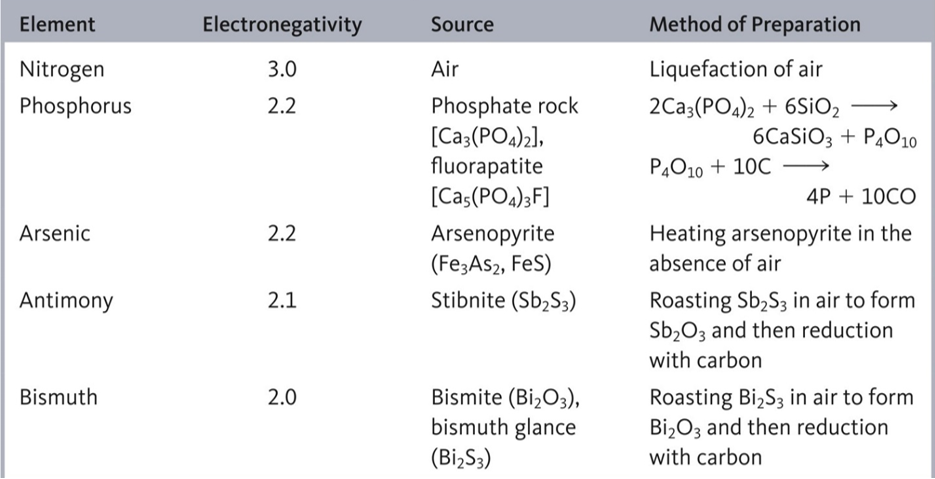
Fig 1: Selected physical properties, sources and methods of preparation of the group 15 elements.