p-Block Elements: Group 15
Oxidation state
The oxidation number or the oxidation states shows the number of electrons that an atom uses or receives when it forms a compound. It denotes the electrons gained by a substance when it is reduced, and the electrons lost when a substance is oxidized. The oxidation number is -ve when the atom gains electrons or shares them with another atom of less electronegativity. The oxidation number is +ve if the atom loses electrons or shares with another atom of more electronegativity. The oxidation number is denoted by Roman numbers, with a plus or minus sign depending on the state of an atom. Each atom in a neutral molecule or charged species can be assigned by its oxidation number depending on the overall molecular charge. Oxidation occurs with an increase in the oxidation number, while reduction occurs with a decrease in the oxidation number. Therefore, the sum of the oxidation number in a compound is equal to its overall charge.
The group 15 elements have 5 valence electrons and they can have variable oxidation state: +3, +3 and +5. The tendency to show -3 state decreases as we gown due to increase in size and metallic character. The stability of +3 state increases down the group whereas that of +5 state decreases due to INERT-PAIR effect Bi shows +5 only with fluorine (BiF5) due to high polarizing power of fluorine.
Elements with higher oxidation state form oxides that are more acidic than elements with lower oxidation state. Likewise, the acidic strength of oxides formed with nitrogen increases from N2O3 to N2O5.Nitrogen compounds disproportionate in acid solutions. Nitrogen has only 4 electrons in its outermost shell (one in s orbital and 3 in p) which is available for bonding, hence it exhibits a maximum covalency of 4. The heavier elements have a vacant d orbital in the valence shell which is used for bonding.
3HNO2 HNO3 + H2O + 2NO
Similarly, for phosphorus almost all intermediate oxidation states disproportionate into +5 and –3 both in alkali and acid. But the +3 oxidation state in case of arsenic, antimony and bismuth becomes increasingly stable and do not undergo disproportionation. With energetically accessible d orbitals, phosphorus and, to a lesser extent, arsenic can form π bonds with second-period atoms such as N and O. This effect is more prominent for phosphorus than for silicon, producing very strong P–O bonds and even stronger P=O bonds.
The first four elements in group 15 also react with oxygen to produce oxide in the +3 oxidation state. P4O6 and As4O6 have cage structures are formed with an oxygen atom at each edge of the P4 or As4 tetrahedron making them behave like typical nonmetal oxides. Likewise, P4O6 reacts with water to form phosphorous acid (H3PO3). Alternatively, Sb4O6 is amphoteric, dissolving in either acid or base. While Bi2O3 behaves as a basic metallic oxide which dissolves in acid giving solutions that contain the hydrated Bi3+ ion. Also, phosphorus and arsenic, form very stable oxides with the formula P4O10and As4O10in the +5 oxidation state.
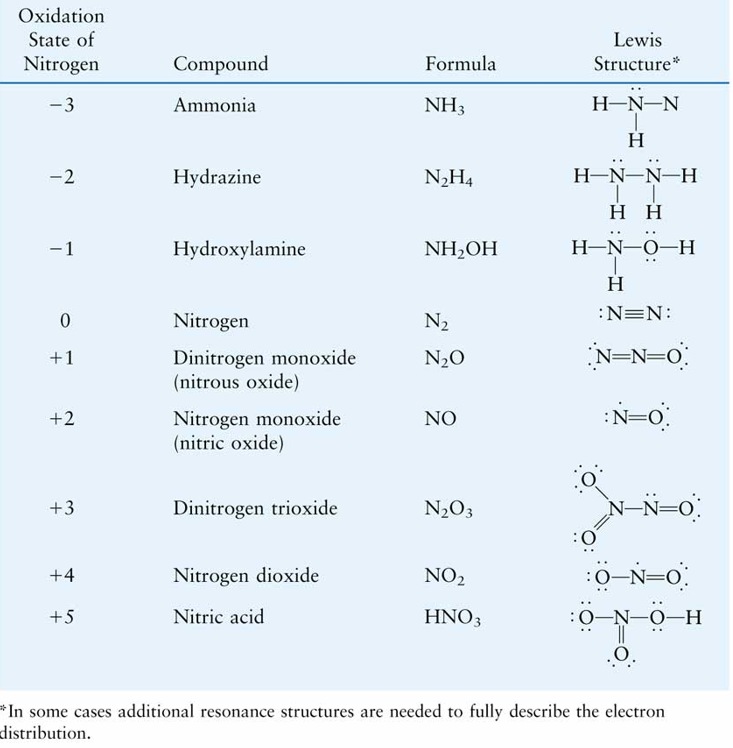
Fig 1 : Common nitrogen compounds and their oxidation states.