p-Block Elements: Group 16
Electronic configuration
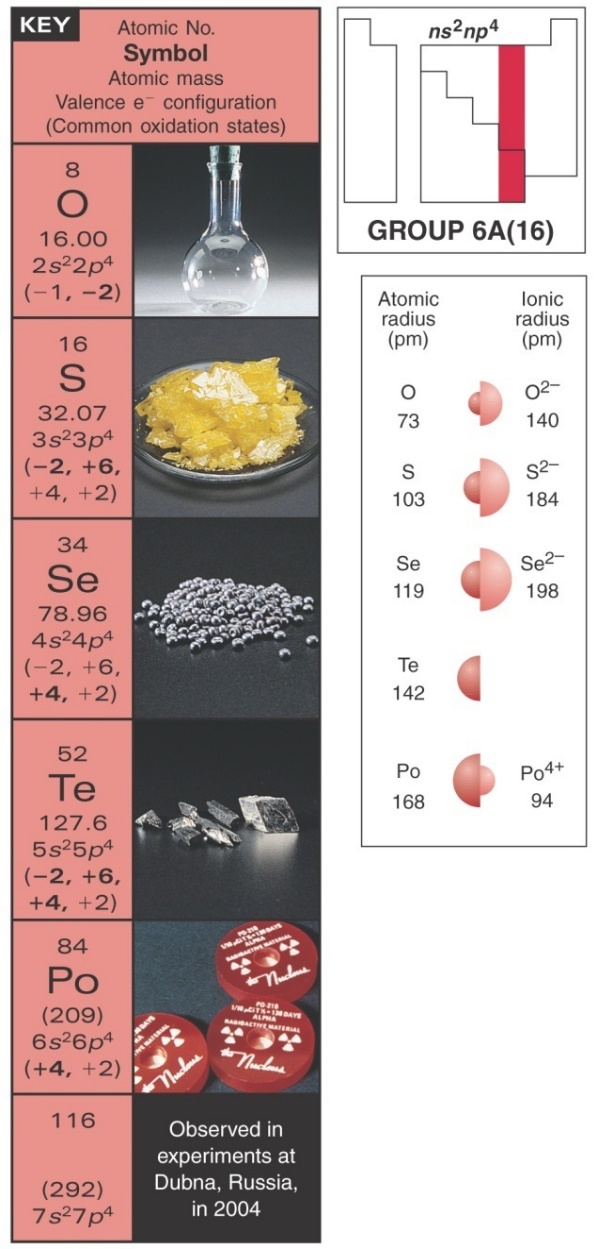
The elements of group 16 have 6 electrons in their outermost valence orbital which accounts for similar chemical properties of the elements. The p sublevel always fills after the s sublevel of a given principal energy level. Likewise, the general electron configuration of group 16 elements is ns2np4. Their ions are doubly charged negative ions have an oxidation state of -2.These elements want to gain 2 electrons to get low energy, stable electron configuration of the noble gases. However, as we go down the group losing electrons becomes more easier than gaining them due to increased shielding effect. Likewise, the metallic nature of elements increases as we go down the group.
Oxygen is smaller in size than other elements in the group but it has high electronegativity and inability to employ an expanded valence shell. It cannot bond with more than 4 elements. Hence it is rarely a central atom in any compound. Alternatively, sulphur shows a higher range of electron bonding having existed in -3 and -2 oxidation state. Also,the oxygen molecule is paramagnetic in nature while other elements in group 16 are diamagnetic. For exampleN2O4 is diamagnetic in nature with even pair of electrons. Same properties work for nitrogen dioxide, NO2 means diamagnetic in nature where there are two terminal oxygens, in this, each electron contributes to two σ bonds. Oxygen shows anomalous behaviour due to the formation of p(pi)-p(pi) bond.
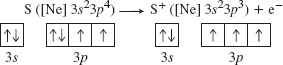
O and S have a fourth np electron, the first to pair up with another np electron, and electron-electron repulsions raise the orbital energy. The fourth np electron is easier to remove because doing so relieves the repulsion’s and leaves a half-filled (stable) np sublevel. This means that the ionization energy decreases from oxygen to Sulphur in the presence of p shell stability. For S, for example,
Selenium can conduct electricity despite being a non-metal. It is a direct gap semiconductor that activates in the presence of visible light. The electrons from p shell in selenium jump to d shell when they gain energy from light and therefore are able to conduct electricity in solar cells. Tellurium is a silver metalloid and also photo conductive owing empty 4d orbital. Electrons are first removed from the ns orbital (5s), then from the (n-1)d orbital (4d).Polonium is more metallic than other elements in group 16 because the valence electrons are furthest from the nucleus and due to shielding effect, the valence electrons are easier to loosen than gaining or sharing more electrons. Likewise, the electronegative character of the elements decreases down the group.
The electronic configuration of each element is given below:
Oxygen: 1s22s22p4
Sulphur: 1s22s2p63s2p4.
Selenium: 1s22s2p63s2p6d104s2p4.
Tellurium: 1s22s2p63s2p6d104s2p6d105s2p4.
Polonium: 1s22s2p63s2p6d104s2p6d10f145s2p6d106s2p4.
Livermorium: [Rn] 5f14 6d10 7s2 7p4