
Phenols are organic hydrocarbon compounds with a hydroxyl group directly attached to an aromatic hydrocarbon compound like Benzene Ring. Phenols are also termed as Phenolic compounds. The molecular formula of phenols is C6H5O6. The common name of phenols is also known as Benzol.
Preparations of Phenol
The preparation of Phenol is either a natural process or a synthetic process. Some of the methods of preparation of phenols are as follows.
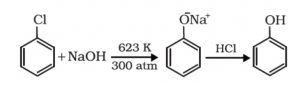
Preparation of phenols from haloarenes:
In this method of preparation of Phenol, the Chlorobenzene is used which is formed by the monosubstitution in the benzene ring. The sodium hydroxide is in the ionic form gets fused in the Chlorobenzene at 623 K and 300 atm. Thus, sodium phenoxide is produced. Further on reaction with acid the O– is attacked by the H+ ion from the acid. The final product is the Phenol hydrocarbon compound.
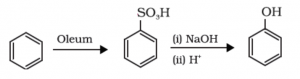
Preparation of Phenol from benzene sulphonic acid:
The Benzene hydrocarbon compound, when reacted with Oleum, gives us the product Benzenesulphonic acid. The Benzene sulphonic acid which is further reacted with molten sodium hydroxide which leads to the formation of sodium phenoxide. The compound thus formed is further passed through the acidification process and the resultant compound is phenol.
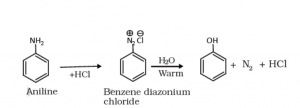
Preparation of Phenol from diazonium salts:
When the Primary Amine is reacted with Nitrous acid at 273 to 278 K, then the diazonium salts are obtained. The diazonium salts thus produced are highly reactive in nature. Thus, when the salts come into the vicinity of warm water, they get hydrolyzed to phenols. These diazonium salts can also be converted to the required products of phenols by treating them with dilute acids.
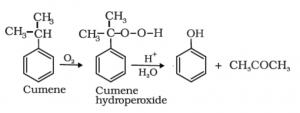
Preparation of phenol from Cumene:
In this method of preparation of phenol, cumene is used. The organic compound of cumene can be obtained by the Friedel craft alkylation process. In this reaction, the Benzene ring is treated with propylene. Thus, the obtained product is cumene. The cumene is further converted into cumene hydroperoxide on reaction with oxygen. This process is done by the method of the Oxidation reaction. The cumene hydroperoxide is further treated with warm water. The warm water hydrolyzes the Cumene hydroperoxide. The end product thus formed is Phenol.
Preparation of phenol from salicylic acid:
Firstly, Salicylic acid is produced by a different process. The acid produced could also be a byproduct from other reactions. The salicylic acid, when reacted with soda lime, undergoes to, the decarboxylation reaction. In the decarboxylation of the salicylic acid, the carboxyl group of the acid is removed. Thus, the obtained result is Sodium phenoxide. The sodium phenoxide has a negative ionic charge on it. On reaction with water or dilute acid the sodium phenoxide is readily attacked with the hydrogen atom. The final product formed is Phenol