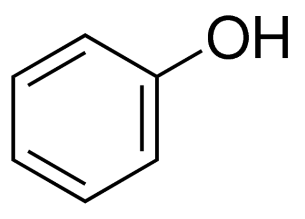
Phenols are organic hydrocarbon compounds with a hydroxyl group directly attached to an aromatic hydrocarbon compound like Benzene Ring. Phenols are also termed as Phenolic compounds. The molecular formula of phenols is C6H5O6. The synthesis of Phenols is Natural as well as artificial.
Physical properties of phenol:
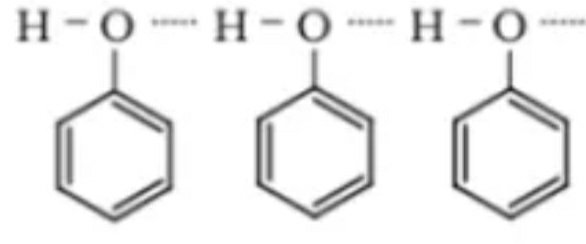
Phenols are aromatic compounds with a hydroxyl group attached to the benzene ring. The phenols are also termed as carbolic acids. The phenols are colorless liquids. But subsequently, due to oxidation in air, they show color. The phenols have OH group hence there are intermediate hydrogen bonding between the oxygen and hydrogen molecule. This bond is formed due to the difference in electronegativity of the atoms. As a result of such hydrogen bonding between different phenol molecules, it becomes difficult to separate the molecules. As a result, the boiling point of the Phenols is more.
The phenols having the oxygen atom have more vicinity towards hydrogen atom. As a result hydrogen bonding is possible with the oxygen atom of phenol and hydrogen atom of water. This dissolution of phenols in water is another physical property of phenols.
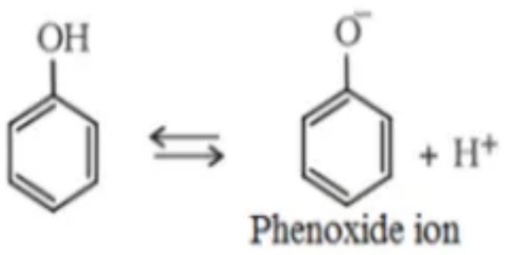
Another famous physical property of phenol is its acidic nature. The phenols have OH atom attached to the sp2 hybridized carbon atoms. As a result, there is a high decrease of electron density on the oxygen atom. This is due to the high electronegativity of the sp2 hybridized carbon atom. This is the reason behind the increase in ionic dissociation of phenols than alcohols. This is the reason for the phenols being more acidic than alcohols.
Chemical properties of phenols:
Along with different physical characteristics of the phenols also have several chemical properties. They are as follows:
Electrophilicsubstitution of phenols.
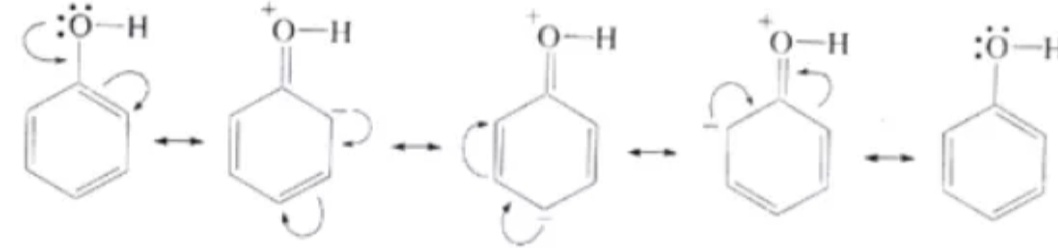
Itis one of the important chemical property of phenol in which there is a rearrangement of electrons along the benzene ring. This makes the phenol compound more attackable by an electrophile. This process makes the benzene ring undergo electrophilic substitution reaction.
Reactions involving breakage of the OH bond of phenols:
Kolbe’s reaction.
In this reaction, the phenol is reacted with NaOH and later the phenoxideion is reacted with CO2 and H2 leading to the formation of Salicylic acid.
Reimer Tiemannreaction.
The Reimer Tiemann reaction is a majorly used process of formation of Salicylaldehyde.
Fries rearrangement.
It is a chemical reaction of phenols converted to phenolic esters and then giving phenolic ketones as the final product.
Acetylation reaction.
It involves acetylation of phenols
Nitration reaction.
This reaction adds the nitro group to the phenol compound.
Halogenation reaction.
Adding of halogens to the phenol compound is halogenation reaction