Haloalkanes are types of hydrocarbons in which hydrogen is replaced by a halogen(group 17 elements)in a normal alkane. The physical properties of haloalkanes are normally like properties of a normal covalent compound. By large functional groups such as carboxyl group or aldehyde doesn’t affect the overall physical properties as halogens are not much reactive. Still, as we move down in the homologous series of haloalkanes group few differences due to difference in atomic masses of the compound can be seen
Physical or chemical properties of any compound depend largely on its mass and the type of intramolecular and intermolecular forces of attraction. Alkyl halides are colourless in its pure form. However, when exposed to light, bromides and iodides develop colour. Also many volatile halogen compounds have sweet smell.
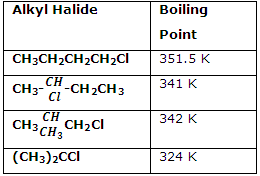
Boiling Point of haloalkanes
There is large electronegativity difference between halogens and carbon resulting in highly polarized molecules. The higher molecular mass and greater polarity as compared to the parent hydrocarbon results in stronger intermolecular forces of attraction (dipole-dipole and van der Waals) in the halogen derivatives.
Boiling Point depends upon the intermolecular forces of attraction and hence the boiling points of chlorides, bromides and iodides are considerably higher than those of the hydrocarbons of comparable molecular mass. As we go down in homologues series of haloalkanes, the forces of attraction becomes stronger due to increase in molecular size and it’s mass, hence the boiling point increases down the homologues series. But the boiling point decreases with branching.Methyl chloride, ethyl chloride and some chlorofluoromethanes are gases at room temperature. Higher members are liquids or solids.
The order of the boiling point of alkyl halides are RI >RBr>RCl> RF
Melting Point of haloalkanes
Melting point of a compound depends upon the strength of lattice structure of a compound. Melting point also follows the same trend as boiling point. An exception to this is para-isomers. The para-isomers have higher melting as compared to their ortho and meta-isomers. It is because of the symmetry of para-isomers. It fits in crystal lattice better as compared to ortho– and meta-isomers.
Density of haloalkanes
Density is directly proportional to the mass of compound, hence down the homologous series, density increases, also fluoro derivatives are lesser dense than chloro derivatives; chloro derivatives are less dense than bromo derivatives and so on.
Solubility of haloalkanes
They are slightly soluble in water. This is because of the relatively larger amount of energy required to break bond between halogen and carbon and the smaller amount of energy released, when bond is formed after dissolution ion and water.