SN1 and SN2 Reactions are used to covert Haloalkanes into alcohols using hydroxide ion in aqueous media. Alcohols can be prepared by substitution of haloalkanes and sulfonic esters with good leaving groups. It is important to choose the right reagents and reaction conditions for the hydrolysis. This is because competitive elimination reactions are possible especially at high temperatures leading to alkenes.
Two kinds of reactions of haloalkanes are seen namely SN1 and SN2 Reaction.
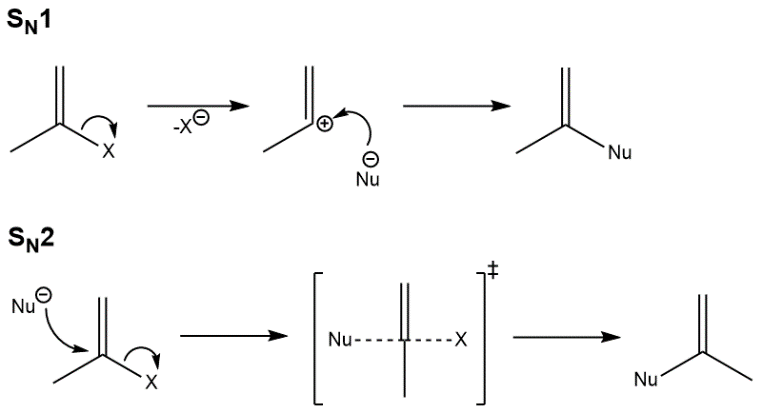
SN1 Reaction
The SN1 reaction is nothing but a substitution nucleophilic unimolecular reaction. The reaction consists of two-step. The carbon-halogen bond breaks heterolytically in the very first step. In this the halogen retains the previously shared pair of electrons. In the second step, the nucleophile reacts rapidly with the carbocation that was formed in the first step.
This reaction follows first order kinetics. Hence, this is named as substitution nucleophilic unimolecular. This reaction takes place in two steps as described below.
Step-1:
- The bond between carbon and halogen breaks due to the presence of a nucleophile and formation of carbocation takes place.
- It is the slowest and the reversible step as a huge amount of energy is required to break the bond.
- Solvation of the compound in a protic solvent break the bond. Therefore this step is slowest of all.
- The rate of reaction is decided only on haloalkane, it doesn’t depend on the nucleophile.
Step-2:
- The carbocation formed in step 1 is attacked by the nucleophileand new compound is formed.
- Since, the rate defining step of the reaction is the formation of a carbocation, hence greater the stability of formation of an intermediate carbocation, more is the ease of the compound undergoing substitution nucleophilic unimolecular or SN1 reaction.
- In case of alkyl halides, because of the high stability of 3o carbocations, 3o alkyl halides undergo SN1 reaction very fast. Hence high reactivity is shown by allylic and benzylic halides towards the SN1 reaction.
SN2 Reaction
SN2 reaction follows second order kinetics. The rate of reaction depends upon both participating nucleophile and the haloalkane. Therefore this reaction is called as substitution nucleophilic bimolecular reaction. The nucleophile attacks the positively charged carbon and the halogen leaves the group in this reaction.
The formation of carbocation and exit of halogen take place simultaneously. This makes it a one-step reaction. Unlike the SN1 mechanism, the inversion of configuration is observed in this reaction. Presence of bulky substituents on or near the carbon atom cause inhibiting effect. This is because this reaction requires the approach of the nucleophile to the carbon bearing the leaving group.
It is favoured mostly by primary carbon, then secondary carbon and then tertiary carbon, completely opposite to SN1 mechanism. Nucleophilic substitution reaction depends on a number of factors. Some of the important factors include.
- Effect of the solvent
- Effect of the structure of the substrate
- Effect of the nucleophile
- Effect of leaving-group.