The ethers are group with R-O-R type of groups. In which the R may be alkyl or aryl groups. Ethers as such due to attached alkyl and aryl groups have a high density of electron. As a result, the ethers behave as electron donating groups. Also, due to these reasons the ethers readily react with the acids.
The nomenclature of ethane doesn’t follow as such any rules. It depends upon the type of alkyl or aryl group connected to the ether. Also, the smaller alkyl group is named as the alkoxy group in the naming of ethers. There are basically two major types ethers namely symmetrical and asymmetrical ethers.
Physical properties of ethers
The ethers as such have many physical properties. The physical properties are as follows:
We know that there are differences in the electronegativity of carbon atoms and the oxygen atoms. Also, the ethers are a group of R-O-R type hence there will be a difference in the electronegativity between the carbon and oxygen atoms. Hence, there will be a net dipole moment between the C-O bond.
The boiling point of ether is comparable to alkanes of similar molecular mass. But, despite the C-O bond polarity, the boiling point of ether is quite lesser than that of alcohol. This is because in alcohols there is hydrogen bonding between the oxygen and hydrogen atoms.
The ethers are readily dissolved in the water. This is because just like alcohol the oxygen of the ether group has a tendency of making hydrogen bonds with the hydrogen of water molecules. As a result, the ethers are miscible in water.
Chemical properties of ether:
The ether is generally not much chemically reactive but still has different chemical properties. Some of the chemical properties of the ether are as follows:
Breakage if the CO bond in ether.
The ethers as such are not very reactive. But, in presence of excess Hydrogen halides, the CO bond of the ether is cleaved. The reactivity of either with hydrogen halides is in such manner HI>HBr>HCl.
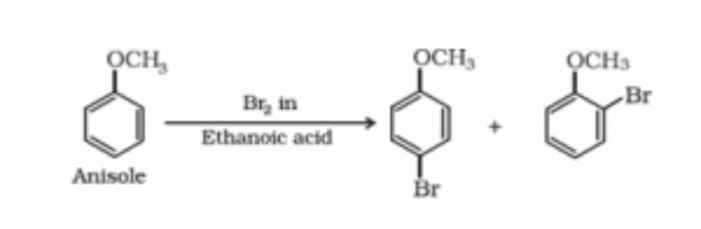
Electrophilic substitution reaction.
As the ethers have alkoxy group in them, they make the ortho and para positions more viable for electrophilic attack by an electrophilic substitution reaction. Some of the famous electrophilic substitution reaction are Bromination reaction and the Friedal craft reaction.
Halogenation reaction
The aromatic ethers undergo halogenation reaction. Most of the halogenation is bromination reaction. It takes place when ether is reacted with an Alkyl halide or acyl halide in the presence of a catalyst or in the absence of player.