Carboxylic Acid is an organic compound which contains a carboxyl functional group. They occur widely in nature and are also synthetically manufactured by humans. Upon deprotonation, carboxylic acids yield a carboxylate anion with the general formula R-COO–, which can form a variety of useful salts such as soaps.
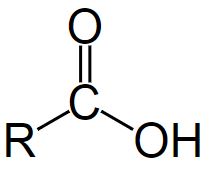
The general formula of a carboxylic acid is R-COOH, were COOH refers to the carboxyl group, and R refers to the rest of the molecule to which this group is attached.
Nomenclature:
IUPAC nomenclature of organic compounds deals with the systematic approach taken for the nomenclature of organic compounds. It functions as per the recommendation of the International Union of Pure and Applied Chemistry(often abbreviated to IUPAC).
Sheer quantity of new discoveries of organic compounds has made the trivial nomenclature of organic compounds highly inconvenient. Because of this the necessity for such a systematic approach of nomenclature arose.
Having talked about its importance, still the IUPAC nomenclature guidelines are not always followed by chemists. This is because some compounds have very long and extremely tedious names as per the IUPAC nomenclature guidelines. These compounds are assigned more trivial names.
The guidelines that must be followed in the IUPAC nomenclature of carboxylic acids are listed below.
- The suffix “e” in the name of the corresponding alkane is replaced with “oic acid”.
- When the aliphatic chain contains only one carboxyl group, the carboxylic carbon is always numbered one. For example, CH3COOH is named as ethanoic acid.
- When the aliphatic chain contains more than one carboxyl group, the total number of carbon atoms is counted and the number of carboxyl groups is represented by Greek numeral prefixes such as “di-”, “tri-“, etc.
- A carboxylic acid is named by adding these prefixes and suffixes to the parent alkyl chain. Arabic numerals are used for indicating the positions of the carboxyl group.
- The name “carboxylic acid” or “carboxy” can also be assigned for a carboxyl substituent on a carbon chain. The name 2-carboxyfuran for the compound 2-Furoic acid is an example for such nomenclature
- Some examples describing the nomenclature of carboxylic acids as per IUPAC guidelines are provided below.
Structure Common name IUPAC name
- HCOOH Formic acidMethanoic acid
- CH3COOH Acetic acid Ethanoic acid
- CH3CH2COOH Propionic acid Propanoic acid
- CH3CH2CH2COOH Butyric acidButanoic acid
- (CH3 ) 2CHCOOHIsobutyric acid 2-Methylpropanoic acid
- HOOC-COOH Oxalic acidEthanedioic acid
- HOOC -CH2 -COOH Malonic acidPropanedioic acid
- HOOC -CH2 -CH(COOH)-CH2 -COOH – Propane-1, 2, 3- tricarboxylic acid