The general formula of a carboxylic acid is R-COOH, where COOH refers to the carboxyl group, and R refers to the rest of the molecule to which this group is attached. In this carboxyl group, there exists a carbon which shares a double bond with an oxygen atom and a single bond with a hydroxyl group.A carboxylic acid contains a hydroxyl group attached to a carbonyl carbon. Due to the electronegativity of the oxygen atom, this functional group can undergo ionization and discharge a proton.
The carboxylate ion, produced from the removal of a proton from the carboxyl group, is stabilized by the presence of two oxygen atoms (through which the negative charge can move). Some of the most common examples of carboxylic acids include acetic acid (a component of vinegar) and Formic acid.
Most of the properties of carboxylic acids are a result of the presence of the carboxyl group. Some of the physical and chemical properties of these compounds are discussed in this subsection.
Physical Properties of Carboxylic Acids
- Carboxylic acid molecules are polar in nature. This is due to the presence of two electronegative oxygen atoms.
- They also participate in hydrogen bonding due to the presence of the carbonyl group (C=O) and the hydroxyl group.
- When placed in nonpolar solvents, these compounds form dimers via hydrogen bonding between the hydroxyl group of one carboxylic acid and the carbonyl group of the other.
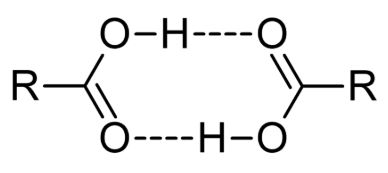
- The solubility of compounds containing the carboxyl functional group in water depends on the size of the compound. The smaller the compound (the shorter the R group), the higher the solubility.
- When compared with the boiling point of the water, the boiling point of the carboxylic acid is generally higher.
- These compounds have the ability to donate protons and are therefore they are a kind of Bronsted-Lowry acids.
- They generally have a strong sour smell. But still their esters have pleasant odours and are therefore used in perfumes.
Chemical Properties of Carboxylic Acids
- Hell-Volhard-Zelinsky reaction can easily halogenate the α-carbon belonging to a carboxylic acid.
- These compounds can be converted into amines using the Schmidt reaction.
- A carboxylic acid can be reduced to an alcohol by treating it with hydrogen to cause a hydrogenation reaction.
- Upon reaction with alcohols, these compounds yield esters.