In the carbonyl, the first carbon atom attached to the functional group is alpha carbon. The alpha hydrogen is made by this carbonyl group i.e. the hydrogen which is present on the alpha carbon is called the alpha hydrogen and due to the resonance stabilization mechanisms, it is slightly acidic. It causes several reactions to occur.In the aldehydes the alpha hydrogen is acidic so, the compounds undergo the many different types of reactions. This acidity is due to its high capacity to withdraw the electrons from the carbonyl group and due to resonance stabilization of conjugate base.
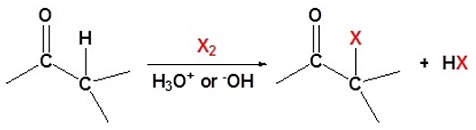
A carbonyl group that is having the alpha hydrogen can undergo the substitution reactions with the halogens. This reaction occurs as carbonyl compounds tend to make enols in the acidic conditions, and enolates in the basic conditions. In these reactions, even the weak bases such as the hydroxide anion are sufficient to make sure the reaction to occur as complete conversion to the enolate is not necessary. The halogens which can be used in these reactions are iodine, bromine, and chlorine.
If no less than one alpha hydrogen is present, then aldol reaction happens in the aldehydes. This reaction occurs in the presence of dilute alkali, which acts as a catalyst in this reaction and helps to make the beta-hydroxy aldehydes. Here the name aldol is derived from the two functional groups such as alcohols, and aldehydes. From the aldol, water is lost and it gives rise to the beta carbonyl compound which is unsaturated and is the product of the aldol condensation, so this reaction is also known as the aldol condensation reaction.
Cross aldol condensation reaction also occurs due to the reactivity of the alpha carbon in the aldehydes and an optimum result of the cross-aldol condensation reaction can be obtained if alpha hydrogen is present in an aldehyde compound and there is no alpha hydrogen in another aldehyde compound. Below is an example of this reaction, with a mixture of propanol and ethanol.