p-Block Elements: Group 16
Preparation properties and uses of sulphur-dioxide and sulphuric acid
Sulphur dioxide
SO2is a colourless gas with a pungent smell which is highly soluble in water. The molecule of SO2 is angular with a resonance hybrid of the two canonical forms:
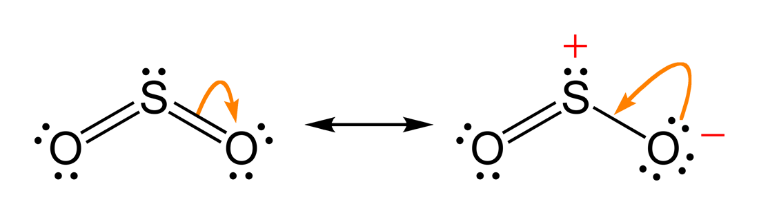
It acts as a Lewis base due to the presence of a lone pair of electrons. It also acts as a reducing agent in both acid and alkaline medium.
Sulphur dioxide passes through water to form a solution of sulphurous acid. It is formed together with a little (6-8
S(s) + O2(g) → SO2 (g)
It is readily generated in the laboratory by treating a sulphite with dilute sulphuric acid.
SO32-(aq) + 2H+ (aq) → H2O(l) + SO2 (g)
SO2 reduces K2Cr2O7 into chromium sulphate
K2Cr2O7+ H2SO4 + 3SO2 → K2SO4 + Cr2(SO4)+H2O
SO2 acts as a reducing and bleaching agent in the presence of moisture.
SO2 + 2 H2O → H2SO4 + 2(H)
During bleaching, SO2 will be oxidised to H2SO4.
Coloured matter + 2(H) → Colourless product
SO2 bleaching is temporary bleaching. Acidic nature decreases from SO2 to PoO2. Bleaching is mainly required for wool and silk. It is also used in the refining of petroleum and sugar.
Sulphuric acid:
Concentrated sulphuric acid is a colourless, dense and oily liquid. It dissolves in water in an exothermic reaction. With wide applications in industry, it is called ‘King of chemicals’. It was also called as‘OIL OF VITRIOL’.It is used in the manufacturing of paints, dyes, detergents, storage battery and laboratory reagents.
The chemical reactions of sulphuric acid occur due to low volatility, strong acidic character, a strong affinity for water and the ability to act as an oxidising agent.
In aqueous solution, sulphuric acid ionises in two steps:
H2SO4(aq) + H2O(l) → H3O+(aq) + HSO4–(aq); Ka1 >10)
HSO4–(aq) + H2O(l) → H3O+(aq) + SO42-(aq) ; Ka2 = 1.2 × 10–2
Sulphuric acidcan be used to manufacture more volatile acids from their corresponding salts
2 MX + H2SO4 → 2 HX + M2SO4 (X = F, Cl, NO3) where M = Metal
Concentrated sulphuric acid is a strong dehydrating agent. Many wet gases can be dried by passing them through sulphuric acid,provided the gases do not react with the acid. Sulphuric acid removes
water from organic compounds; it is evident by its charring action on carbohydrates.
Hot concentrated sulphuric acid is used owing to its moderately strong oxidizing properties. The acidic strength of sulphuric acid is intermediate between phosphoric and nitric acids. Both metals and non-metals are oxidised by concentratedsulphuric acid, which is reduced to SO2.
Cu + 2 H2SO4(conc.) → CuSO4 + SO2 + 2H2O
3S + 2H2SO4(conc.) → 3SO2 + 2H2O
C + 2H2SO4(conc.) → CO2 + 2 SO2 + 2 H2O