p-Block Elements: Group 16
The industrial process of manufacture, properties and uses
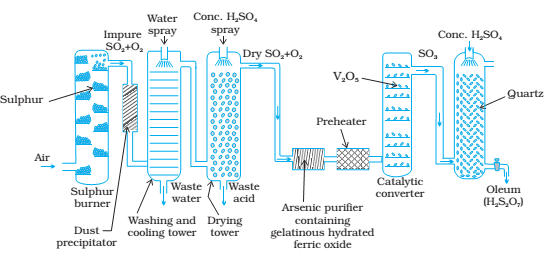
Sulphuric acid is one of the oldest and most important industrial chemicals worldwide. Sulphuric acid is manufactured at industrial scale by Contact Process.
It involves three steps:
1) Combustion of liquid sulphur or sulphide ores in the air to generate SO2.
At first, the air is dried with sulphuric acid followed by injection of pure liquid sulphur in the burner under pressure, 2 atm. The reaction takes place at 600°C. The reactor cools down and steam is used to pre-heat and vapour from the absorption towers used to cool the reactor. This step minimizes the cost of manufacturing to maximize profit.
S + O2 SO2
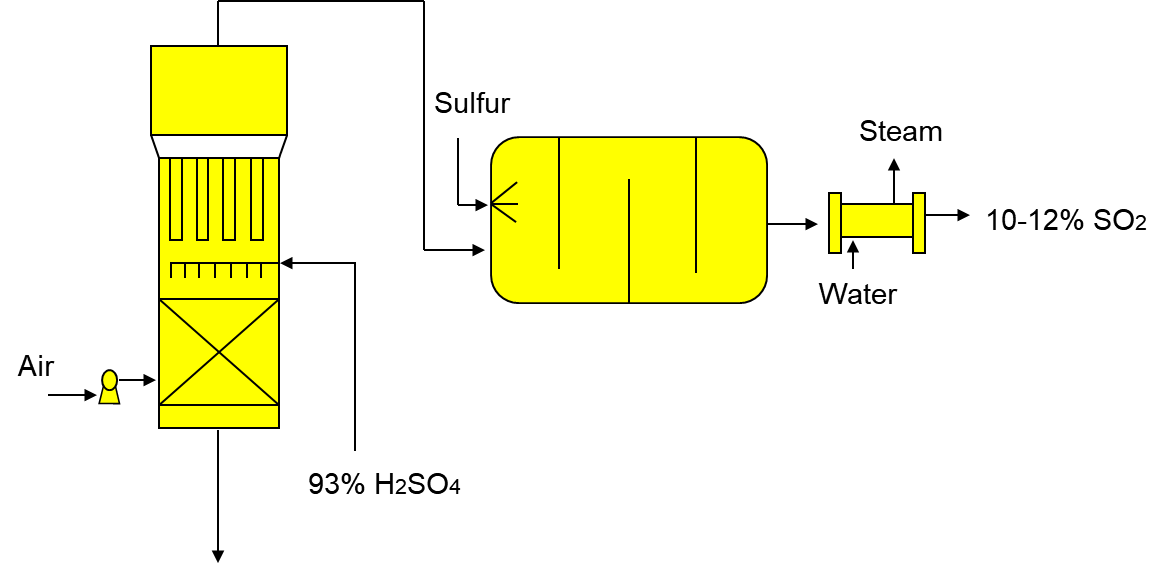
Fig : The Furnace.
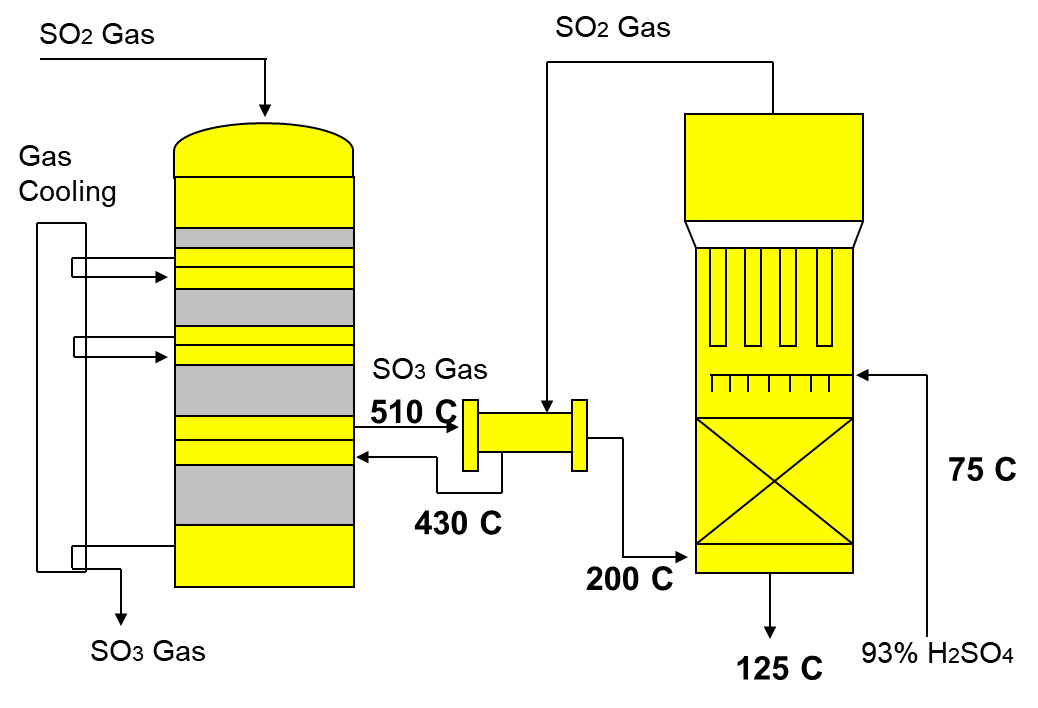
Fig : The converter with temperature profile.
2) Conversion of SO2 to SO3 by the reaction with oxygen in the presence of catalyst V2O5.
This is an exothermic reaction with multiple steps. This reaction is reversible and reaches an equilibrium. It is also an exothermic reaction and the temperature will rise to over 600°C. The mixture is continuously cooled to 400°C between each tray. As the temperature increases the equilibrium shifts to the left and reduces the yield of production of SO3. To counter this the gases are cooled slightly before they pass over the next layer of catalyst
2SO2 + O2 (V2O5) 2SO3
3) Absorption of SO3 in to give oleum (H2S2O7).
Sulphur trioxide dissolves in water to form sulfuric acid. However, it is violently exothermic and usually results in a mist of sulfuric acid droplets that are very difficult to control. In practice, the sulphur trioxide dissolves almost completely and is bubbled through concentrated sulfuric acid (that contains relatively little water) to form 98% sulfuric acid, known as Oleum (H2S2O7)
- SO3(g) + H2SO4(l) H2S2O7(l)
- H2S2O7(l) + H2O(l) H2SO4(l)
Most of the “waste” heat is recovered and used to heat water, in this way much of the energy can be reused. The double absorption method is used to prevent SO2 emissions. After the first round of processing through the converter, any SO2 that was not converted into SO3 can be collected and passed back through.
The fertilizer market captures 50-65% of all the sulphuric acid produced in the world. Second is the organic chemical industry where sulphuric acid is used to produce plastics and synthetic fibres. Production of a chemical called titanium dioxide consumes large quantities of sulfuric acid. TiO2 is a white pigment used in paints, plastics and cosmetic industry. It is also used for pickling ferrous and non-ferrous materials in the metal industry. The recovery of copper, nickel, and zinc from low-grade ores also required sulphuric acid. Other uses include paper, dyes, drugs
Sulfuric acid is a strong acid, dehydrating agent and an oxidant. Sulfuric acid is diprotic. In a reaction with water, the first proton will be donated to form the hydronium ion and HSO4–. Sulfuric acid dehydrates sugar into water and carbon and will dehydrate copper sulphate as shown below.
Uses of sulphuric acid
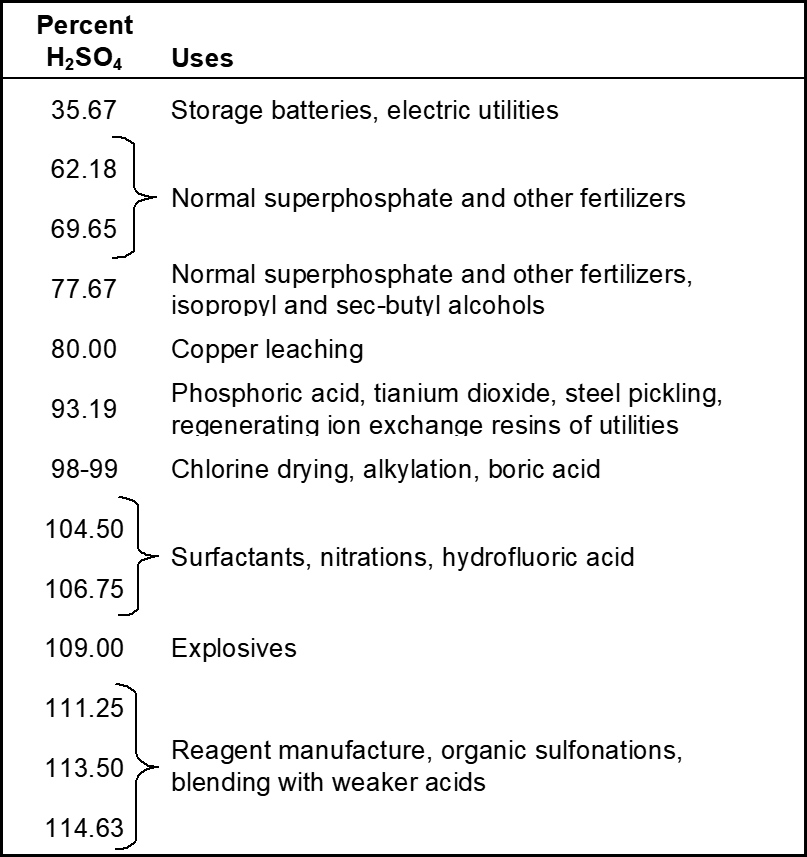
Fig : Uses of sulphuric acid.