Electrophilic substitution reaction is nothing but the reaction in which an electrophile substitutes another electrophile in an organic compound. Electron seeking species are known as an electrophile. Haloarenes undergo electrophilic reactions of benzene ring. These are halogenation, nitration, sulphonation, and Friedel-Crafts reactions. We will discuss about them one-by-one, but let’s first understand the behaviour of reaction of Haloarenes towards the attack of an electrophile:
- Due to –I effect that is the electron withdrawing nature of halogen, benzene ring gets somewhat deactivated towards electrophilic substitution reaction.
- Due to its various resonating structures, there’s an excess of electron or negative charge over ortho- and para- positions of the ring than the meta- position. Thus haloarenes are p- and o- directive towards electrophilic substitution reaction.
Haloarenes are somewhat deactivated than normal benzene ring towards the electrophilic substitution reaction due to the above reasons. Thus these reactions occur slowly and require more drastic conditions as compared to those in benzene.
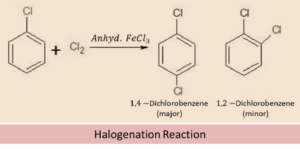
Halogenation
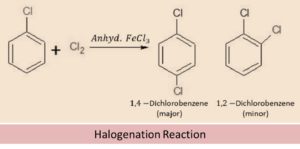
From the above equation of Haloarenes we can see that, when a haloarene comes in the vicinity of chlorine in the presence of ferric chloride as solvent, then the chlorine molecule develops a polarity within itself and the chlorine with a slightly positive charge acts as electrophile (electron seeking) and attacks the electron rich ortho and para position of haloarene. Both ortho and para compounds are formed out of which para isomer is the major product and ortho isomer is the minor product.
Nitration
In this reaction, first NO2 is formed from nitric acid which is initiated by the presence of sulphuric acid; NO2 has an electrophilic centre over N due to the presence of two electronegative oxygen atoms in the molecule. NO2attacks the electron rich ortho and para positions, out of which we get para isomer as the main product and ortho isomer as the minor product.
Sulphonation
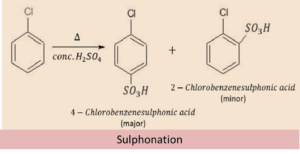
Sulphur Trioxide formed from sulphuric acid acts as an electrophile. SO3 attacks the electron rich ortho and para positions of the haloarene, out of which para isomer is obtained as the major product and ortho isomer as the minor product.
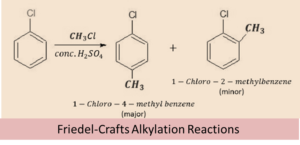
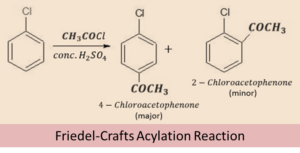
Friedel-Crafts reaction
Alkyl and acetonic group act as electrophile in this reaction, due to the presence of positive charge over the carbon atom. These groups attack the electron rich ortho and para positions of the haloarene, in which the para isomer of the product is obtained as the major product and ortho as the minor product.