p-Block Elements: Group 17
Interhalogen compounds
Interhalogen compounds are produced by reacting halogens with each other. Most interhalogen compounds resemble the properties and behaviours that are intermediates of those of the two-parent halogens. Inter-halogens can be grouped into four categories: XY, XY3, XY5, and XY7. Here X halogen atom is more electropositive and larger in size than Y halogen atom. The oxidation state of atom X in XY, XY3, XY5, and XY7 molecules is equal to+1, +3, +5 and +7 respectively. As the ratio between the radii of X and Y atoms increases, the number of halogen atoms per molecule increases. These compounds are easiest to form when Y is fluorine. Iodine is the only halogen that forms an XY7 interhalogen compound with fluorine that allows least electronegative and most electronegative compound among halogens to combine.
Consider the following examples:
Bromine reacts with chlorine to produce bromine chloride: BrCl, which is a gas at room temperature.
Chlorine reacts with fluorine to form chlorine trifluoride.
The stability of XY type inter-halogen compounds with respect to disproportionation is in the order:ClF>ICl>IBr>BrCl>BrF. The Lewis acid strength of these compounds decreases in the order: ICl>>BrCl>IBr> I2. All polyhalides are coloured compounds and depth of colour increases with the increase of the atomic number of halogen atoms. Polyhalides are highly soluble in water and get dissociated in water. The stability of metallic trihalides of MX3 type having the same cation in the same oxidation state is in the order: MI3 > MBr3> MCl3. And the stability of metallic trihalides, having the same trihalide anion and different cation increases with the increase in the size of the cation. For example:NaI3< KI3< RbI3< CsI3.ClF3 and BrF5 are extremely reactive compounds and they are highly unstable. ClF3 is so reactive that wood, asbestos, and even water spontaneously burn in its presence. These compounds are excellent fluorinating agents, which tend to react with each other to form positive ions such as ClF2+ and BrF4+ and negative ions such as IF2– and BrF6–.
The structures of various interhalogens conform based on the VSEPR model. XY compounds undergo sp3 hybridization with lineal shape. For XY3the hybridization is sp3d. It is a T-shaped with 2 lone pairs sitting in equatorial positions of a trigonal bipyramid. For XY5the hybridization is sp3d2. It is a square pyramid with the unpaired electrons sitting in an axial position of an octahedral. XY7 is a pentagonal bipyramid with sp3d3hybridisation.
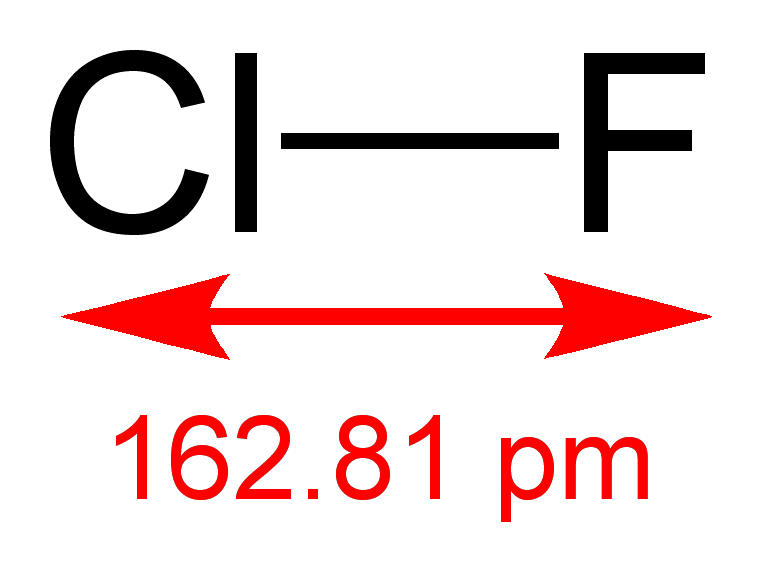
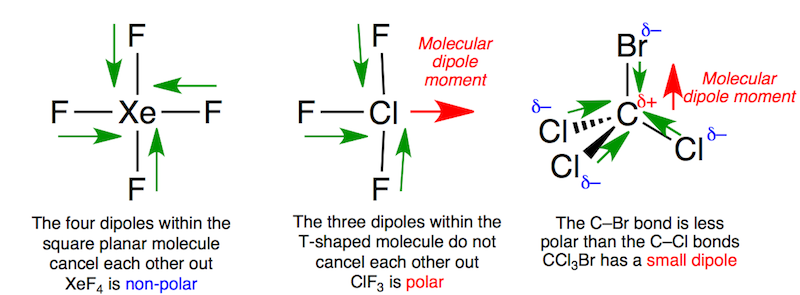

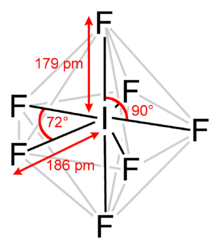
Fig : Some structural examples of interhalogen compounds.
A lot of these compounds are unstable solids or fluids at 298K. A few other compounds are gases as well. On the other hand, bromine trifluoride and iodinetrifluoride are solid and liquid respectively. These interhalogen compounds are very reactive, covalent and diamagnetic in nature. This is because they have bond pairs and lone pairs. One exception to this is fluorine because the A-X bond in interhalogens is much weaker than the X-X bond in halogens, except for the F-F bond. 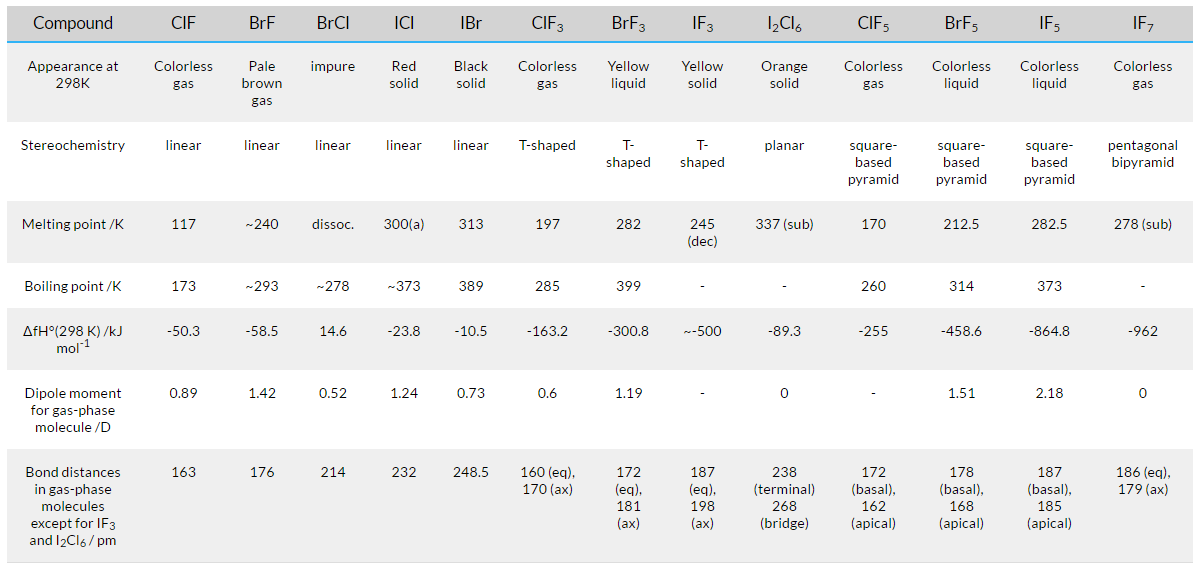
Fig 2: Properties of interhalogen compounds.