p-Block Elements: Group 17
Oxoacids of halogen
The fluorine atom is highly electronegative and compact in size. Therefore, it can only form a single oxoacid. HOF is fluoric(I) acid or hypofluorous acid. Alternatively, the other elements of the halogen family produce several oxoacids with multiple oxygen atoms. The focal halogen molecule in these oxoacids is formed in sp3 hybridisation. This means that the central atom in the oxoacids obtains sp3 hybridized with additional d orbital hybridization depending on the number of oxygen atoms. Oxoacids have essentially one X-OH bond that may or may not induce hydrogen bonds and they have multiple X=O bonds present in them. In the majority of these oxoacids, double bond structures of “X = O” bonds are available to provide some form of stability to the compounds. Examples of hypohalous acids are for fluorine, chlorine, bromine and iodine are hypofluorous acids, hypochlorous acid, hypobromous acid and hypoiodous acid respectively. The halogen obtains the oxidation state of +1 in hypohalous acids while halous acid has +3, halic acid has +5 and lastly, perhalic acid has +7 oxidation state. Examples of each type are shown below.
| Halic(I) acid (Hypohalous acid) | HOF (hypofluorous acid) | HOCl (Hypochlorous acid) | HOBr (Hypobromous acid) | HOI (Hypoiodous acid) |
| Halic(III) acid (Halous acid) | – | HOClO
(chlorous acid) |
– | – |
| Halic(V) acid
(Halic acid) |
– | HOClO2
(chloric acid) |
HOBrO2
(bromic acid) |
HOIO2
(iodic acid) |
| Halic (VII) acid (Perhalic acid) | – | HOClO3 (perchloric acid) | HOBrO3 (perbromic acid) | HOIO3
(periodic acid) |
Chlorine can formall four types of oxoacids. They are HOCl (hypochlorous acid), HOClO (chlorous acid), HOClO2(chloric acid) and lastly HOClO3 (perchloric acid). Chlorine reacts with the OH– ion to form chloride ions and hypochlorite (OCl–) ions.
This is called a disproportionation reaction where one-half of the chlorine atoms are oxidized to hypochlorite ions while the other half is reduced to form chloride ions.
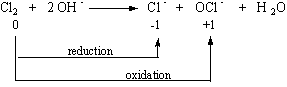
When the solution is heated up, this reaction gives a mixture of the chloride and chlorate (ClO3–) ions.
Under carefully controlled conditions, it is possible to convert a mixture of the chlorate and hypochlorite ions into a solution that contains the chlorite (ClO2–) ion.
Oxyanions of the halogens are named by using the endings –ite and –ate to indicate low and high oxidation numbers and the prefixes hypo– and per– to indicate the very lowest and very highest oxidation numbers as discussed previously. Each of these ions can be easily converted into an oxyacid. These oxyacids are named by replacing the –ite ending with –ous and the –ate ending with –ic respectively.The last member of this class of chlorine compounds, the perchlorate ion (ClO4–), is produced by electrolyzing solutions of the chlorate ion.
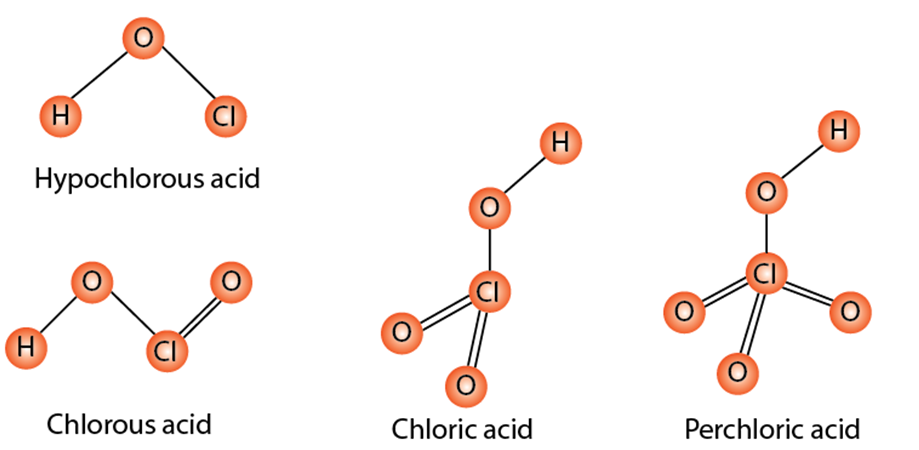
Fig : oxoacids of chlorine
Bromine forms HOBr (hypobromous acid), HOBrO2(bromic acid) and HOBrO3 (perbromic acid).
Iodine forms HOI (hypoiodous acid), HOIO2 (iodic acid) and HOIO3 (periodic acid). Astatine is an unstable radioactive element whose oxoacids are difficult to prepare in laboratory conditions.