Amines are basically the derivatives of the Ammonia (NH3). These derivatives are formed by the replacing of the hydrogen groups from the Ammonia. The replacing of hydrogen atom in the ammonia is done by either the Alkyl groups or the Aryl groups respectively. The Amines as such behave like base in nature. This is because the Nitrogen atom itself has 2 valence electrons in its orbital. Also, there are Alkyl groups, or the Aryl groups or the Hydrogen atoms attached to the compound. As a result the amine group behaves as a the base and electron donating group.
Classification:
The classification of amines can be done on the basis of the number of Hydrogen atoms are replaced by the Alkyl or the Aryl groups in the parent Ammonia. The Amines are classified differently from the alkyl halides or the alcohols. The main reason behind this is that thee nitrogen atom in the amines form three neutral single bonds and have a lone pair with it. A maximum of three hydrogen atoms of the ammonia could be replaced by other groups. But, by the further treatment with a Halide the lone pair could be removed and one more group could be added.
The amines are classified as Primary, Secondary, Tertiary and Quaternary Amines. The primary, secondary, tertiary and quaternary amines are also known as 1˚, 2˚, 3˚, 4˚ amines respectively.
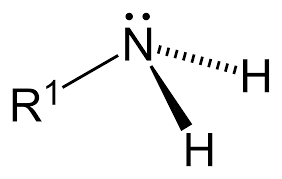
The Primary Amine is also known as 1˚ amines. The primary amines are derived from the parent Ammonia compound by replacing one of the hydrogen atoms of ammonia. The primary amine is also known as Amino. This amine is highly basic in nature as it has two hydrogen atoms linked to it along with an alkyl group. Also, there are two valence electrons on nitrogen making the compound highly basic.
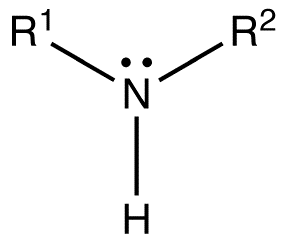
The Secondary amines are also known as 2˚ amines. The secondary amines are derived from the Ammonia compound, by replacing two hydrogen of ammonia compound. The two hydrogen atoms of the compound are either replaced by the Alkyl groups or Aryl groups respectively.
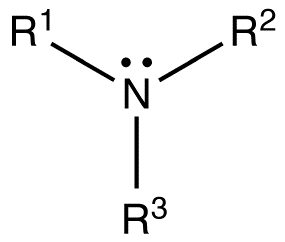
The tertiary amines are also known as 3˚ amines. These amines are formed by the replacement of all the three hydrogen atoms of ammonia. The groups replacing the hydrogen atoms would be alkyl or aryl groups. Since, there is no hydrogen atom left in the compound it has lower boiling point than other derivatives of amines. This is because they cannot make hydrogen bonds with themselves.
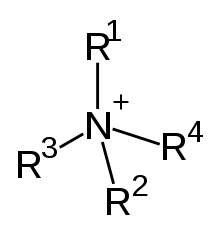
The quaternary amines are also known as 4˚ amines. The quaternary amines actually exist as quaternary ammonium salts and bonds with halides.