p-Block Elements: Group 18
Electronic configuration
All noble gases have full outer electron shells and do not need to gain, lose or share electrons. This means that:
- They are very stable and the most unreactive (or inert) of all the elements.
- They do not normally form bonds with other elements.
- They are monatomic, which means they exist as individual atoms. Most other gases are diatomic
Noble gases are found in group 8A or 18 or 0. The elements are designated as noble elements because they are non-reactive and very stable at room temperature. Most of the noble gases do not tend to form compounds except the heavier gases such as Xenon.The general electronic configuration of group 18 elements is ns2np6 except for helium which has 1s2. Eventhough helium has 2 electrons it is grouped with the elements containing 8 electrons in the valence shell. The second shell is in full occupancy which results in the stable electron arrangement where all the electrons are paired and occupy the lowest energy level giving it an inert property. The electronic configurations of all the Noble gases are listed below.
Helium 1s2
Neon [He] 2s2 2p6
Argon [Ne] 3s2 3p6
Krypton [Ar] 3d10 4s2 4p6
Xenon [Kr] 4d10 5s2 5p6
Radon [Xe] 4f14 5d10 6s2 6p6
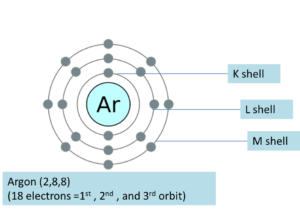
Figure 1: Arrangement of electrons in Argon atom.
Consider the electron configuration of argon which has all but the 3d orbitals filled. When the electronic configuration of Argon is written, the first two electrons are placed in the 1s orbital.1s orbital can only hold two electrons, so the next 2 electrons for Argon go in the 2s orbital. The following six electrons find occupancy in the 2p orbital. As we are aware that the p orbital can hold up to six electrons, we shall put six in the 2p orbital and then further two electrons in the 3s. 3s orbital also goes full in occupancy as we move onto 3p orbital where we’ll place the remaining six electrons. Therefore, the Argon electron configuration can finally be written as 1s22s22p63s23p6.The 3d orbitals are higher in energy so the electrons don’t jump up to that shell and find themselves paired in a stable octet of the 3rd shell. Argon is stable with the electron configuration of the first second and most of the third energy level being completely filled. This pattern is followed by the next elements down the group 18 (VIIIA) making all the noble gases to have a stable octet in the outer most shell.
The ionization energies of the noble gases decrease with increasing atomic number and atomic size. The atomic size is measured by analyzing the van der Waals radius of the gases. Only highly electronegative elements are able to form stable compounds with the noble gases in positive oxidation states and avoiding being oxidized themselves. A potent oxidant is needed to oxidize noble gases and form compounds in positive oxidation states. Similar to the properties of heavier halogens, xenon and perhaps krypton are able to form covalent compounds with F, O, and possibly Cl. These elements can possibly have formal oxidation states (+2, +4, +6, and possibly +8). Xenon has a high affinity for fluorine and oxygen owing to its low electronegative tendency. It can form stable compounds that contain xenon in even oxidation states up to +8.