p-Block Elements: Coordination compounds Bonding
The nature of bonding in complexes can be explained using several theories and some of them are discussed below:
Valence Bond Approach (VBA)
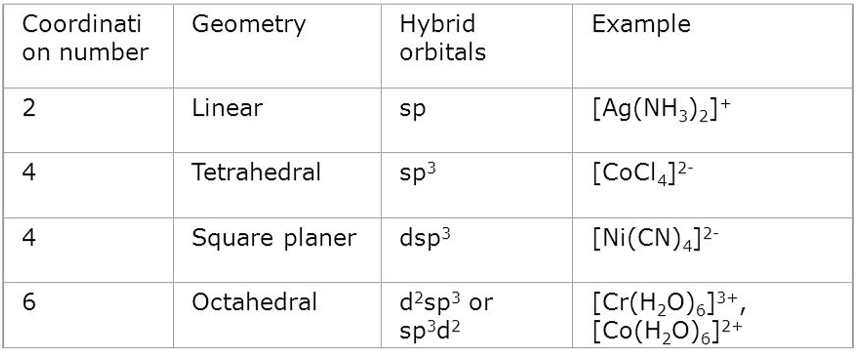
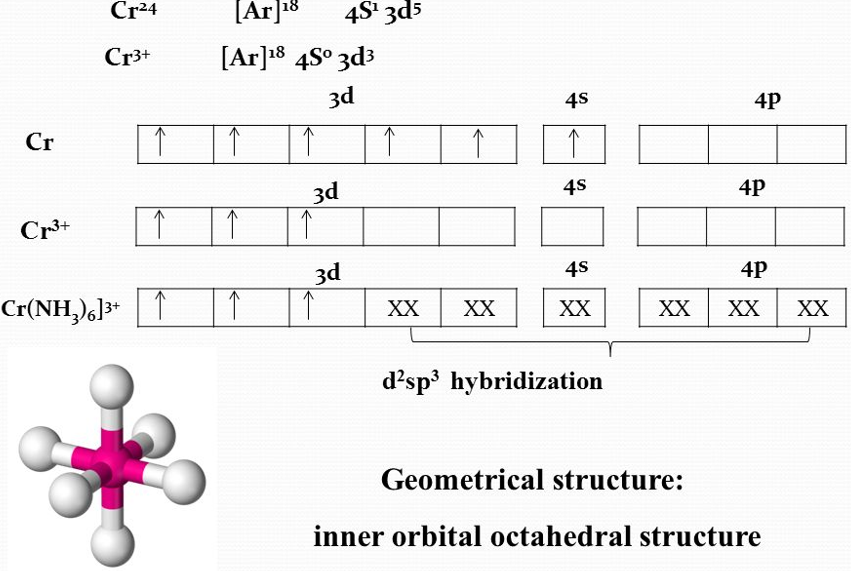
According to this approach, the formation of a complex is a reaction between a Lewis base (ligand) and a Lewis acid (metal or metal ion) happens in combination with a coordinate covalent bond between the metal and ligand. The metal atom or ion under the influence of ligands can use (n-1)d, ns, np or ns, np, nd orbital for hybridization to yield a set of equivalent orbitals of definite geometry. These hybridized orbitals can overlap with those of ligand orbitals that can donate an electron pair for bonding.
Example:
Crystal Field Approach (CFA)
The crystal field approach applies an electrostatic model for the metal-ligand bond that is formed by the electrostatic interactions between the metal ion and the ligand. The transition metal (central metal atom) is regarded as a positive ion and is surrounded by negative or neutral ligands which have a lone pair of electrons. If the ligand is neutral like NH3, the negative end of the dipole in the molecule is directed towards the metal atom. The electrons on the central metal atom are under repulsive forces from the ligands, hence they occupy the d orbital furthest from the direction of the ligands.
In the crystal field approach, the following assumptions are made.
1. Ligands are treated as point charges.
2. No interactions take place between metal orbitals and ligand orbitals.
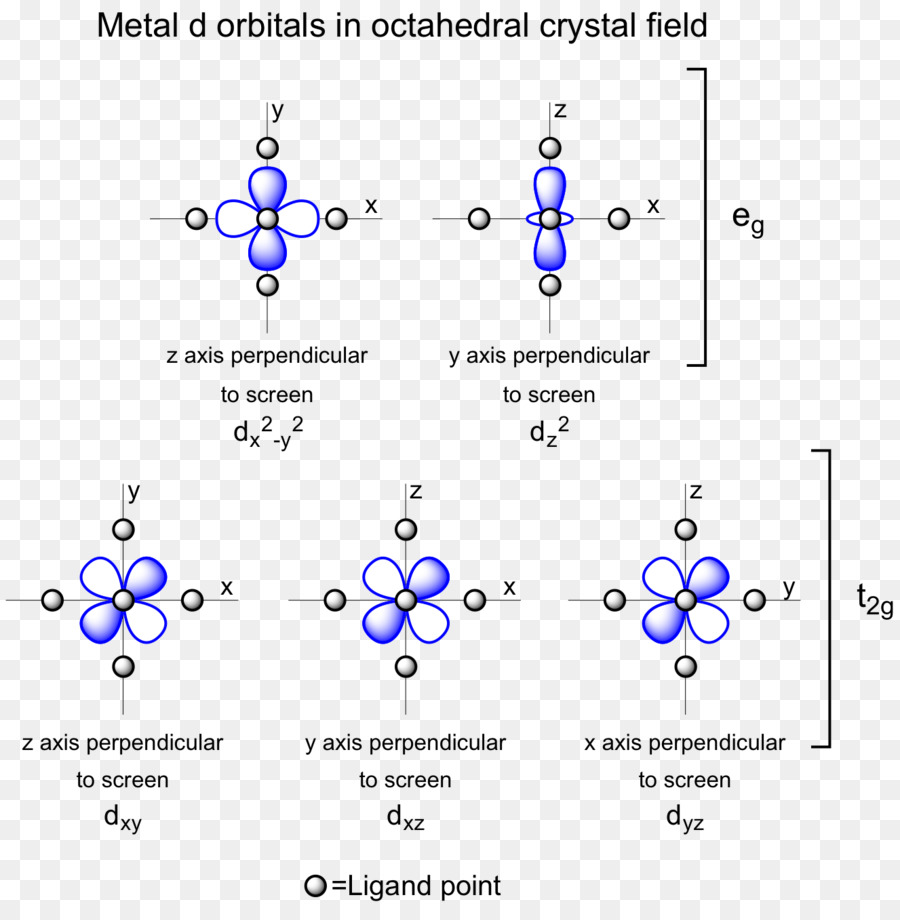
3. The d orbitals on the metal atom which were of the same energy (degenerate) in an isolated atom, have their degeneracy removed by the ligands when a complex is formed. It results in the splitting of d orbitals. The pattern of splitting depends on the nature of the crystal fields induced by ligands.
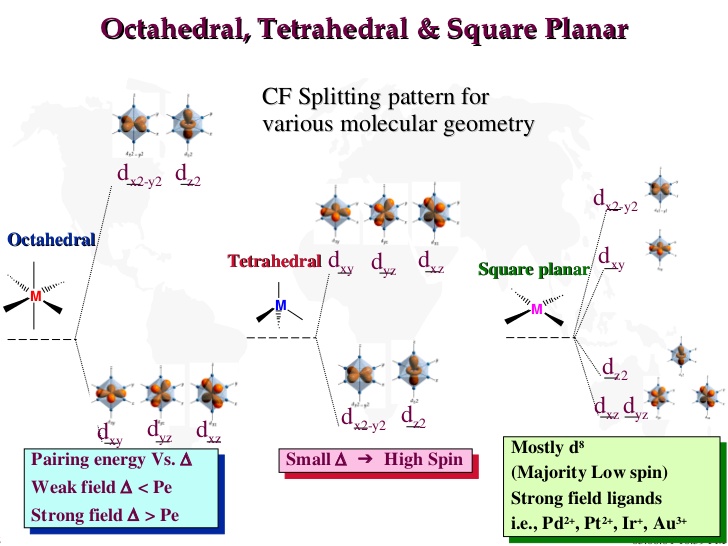
Ligand Field Approach (LFA)
Ligand field theory considers the effect of donor atoms on the energy of d orbitals in the metal complex which depends on the coordination geometry of the ligands.
Sigma bonding
Overlap of metal orbitals and the linear combination is ligand group orbitals leads to the formation of molecular orbitals. The s orbitals transform to a1g, set of p orbitals as t1u, while five d orbitals lose their degeneracy to form eg and t2g.
- Spherical a1g orbitals can overlap with ligan group orbitals on all axes.
- t1uand eg have lobes along the bond direction and participate in bonding.
- t2g has lobes directed between the bonding axis which yield no overlap with ligand orbitals.
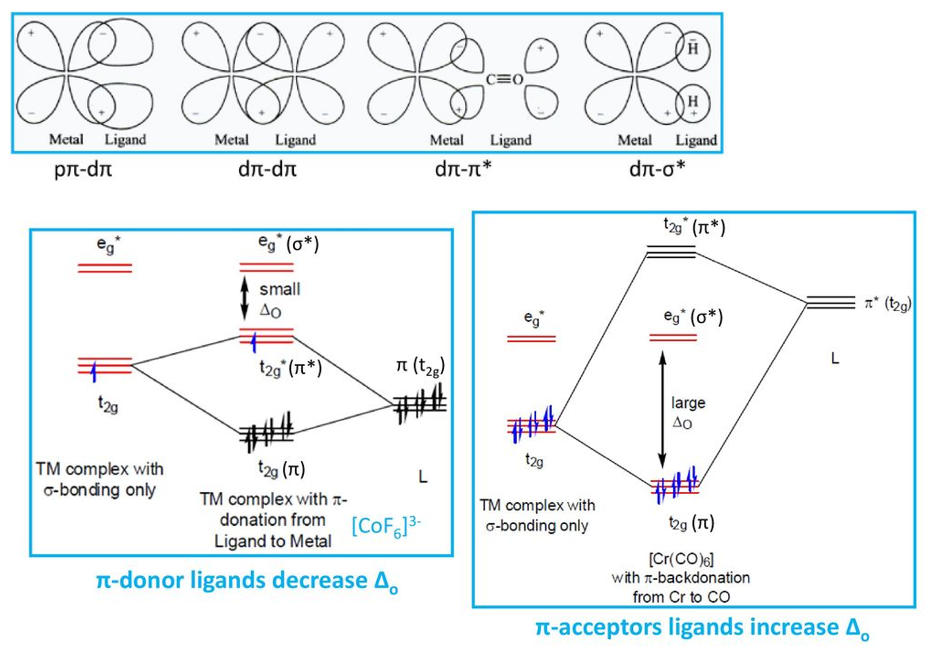
Pi bonding
Metal t2gorbitals which are non-bonding in the presence of σ donor ligand can form π bonds with pπ, dπ, dπ* and dσ* orbitals. The strongest π interactions are between a metal dxy orbitals and a ligand π* orbital. Ligands that can behave as both π acceptors and π donors (such as CO, CN-) presents the π acceptor nature to dominate.
Molecular orbital approach
In contrast to crystal field theory, molecular orbital considers the covalent nature of the metal-ligand bond interaction. The combination of atomic orbital wave functions generates equal numbers of bonding and antibonding molecular orbitals. The bonding MO is always lower in energy than the antibonding MO.
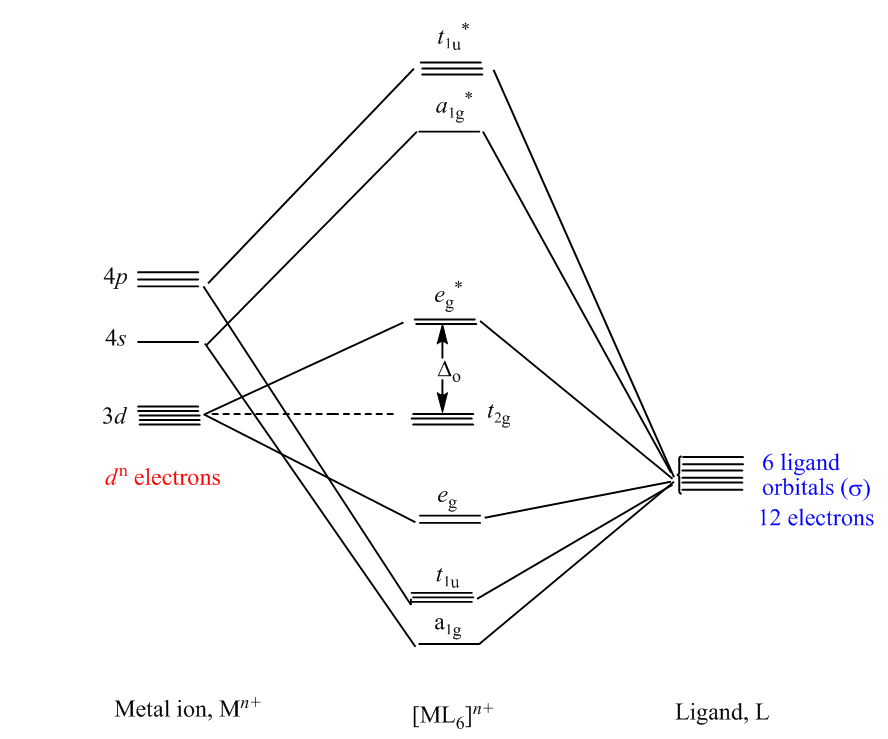
Fig :MO diagram for the σ-bonded octahedral metal complex
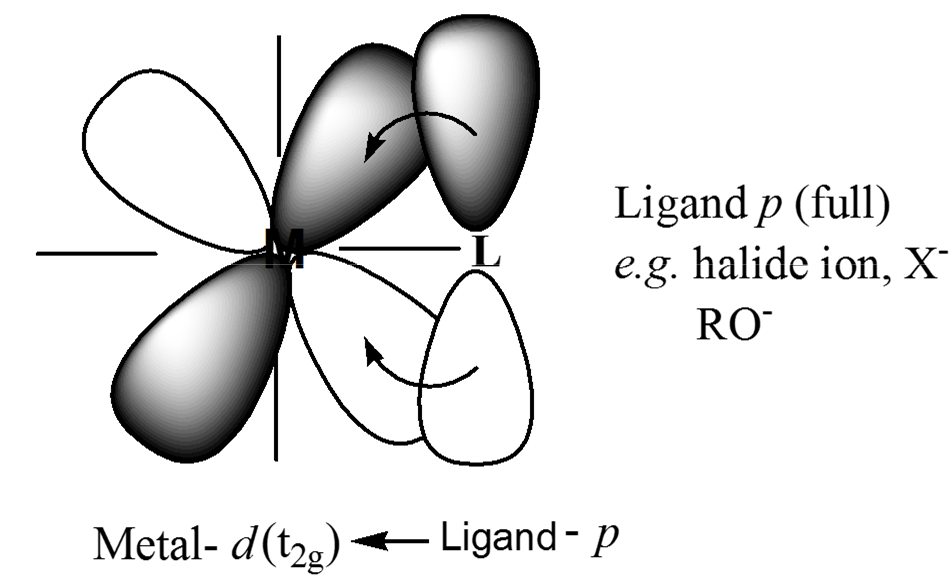
Metal-ligand π-bonding interactions
t2g orbitals (dxy, dxz, dyz) are non-bonding in an σ-bonded octahedral complex. the ligands of π-symmetry overlap with the metal t2g orbitals to form metal-ligand P-bonds. π-unsaturated ligands such as CO, CN– or 1,10-phenanthroline or sulfur and phosphorus donor ligands (SR2, PR3) with empty t2g–orbitals have the correct symmetry to overlap with the metal t2gorbitals.

π-acceptor interactions lower the energy of the non-bonding t2g orbitals and increasing the magnitude Doct. This explains why π-acceptor ligands like CO and CN– are strong field ligands, and why metal carbonyl and metal cyanide complexes are generally low-spin.
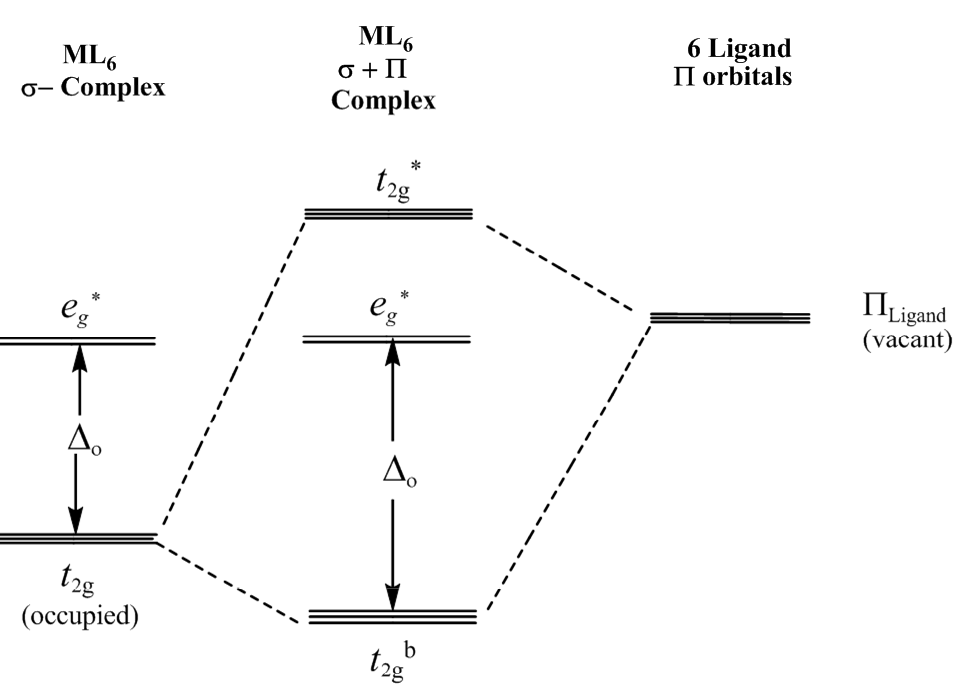
The π interactions involving P-donation of electron density from filled p-orbitals of halides (F– and Cl–) and oxygen donors, to the t2g of the metal, can have the opposite effect of lowering the magnitude of Doct. The t2gelectrons of the σ-complex that are derived from the metal d orbitals, are pushed into the higher t2g* orbitals and become antibonding. This has the effect of lowering Doct.