In the class of organic compounds, carbohydrates are the most abundant, and they are the products of photosynthesis, formed by the endothermic reductive condensation of the carbon dioxide in the presence of the pigment chlorophyll and light energy. They are the main source of energy for metabolism for animals and plants or the living organisms which are dependent on the plants for getting the food. Carbohydrates having a relatively small size are categorized as the sugars and on the basis of the C=O functional group, carbohydrates are further classified into the aldoses and ketoses.
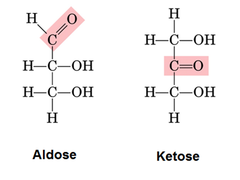
Aldoses
For the carbohydrates, to be categorized as aldose It must have an aldehyde group. Aldose is a monosaccharide and it has only one aldehyde group per molecule. Diose glycolaldehyde is the simplest aldose that is having only two carbon atoms. In the aldoses, at least one asymmetric carbon center is present, so the aldoses that have three or more than three atoms exhibit the phenomenon of stereoisomerism. The examples of aldoses are xylose, ribose, allose, lyxose, threose, glyceraldehyde, glucose, idose, galactose, talose, mannose, altrose, and arabinose. All these aldoses are having one aldehyde group but they differ from each other in the number of carbon atoms in the carbon skeleton. Aldoses that have the steroegenic centers can exist either as L-form or as D-form and their form can be determined on the basis of chirality of the penultimate carbon.
Ketoses
The monosaccharide having the carbonyl function on the inner atoms of the carbon chain is classified as the ketose. Here the dihydroxyacetone is not a sugar but it is counted as ketose analog of the glyceraldehyde. Commonly, at C-2 carbonyl group is attached and the carbonyl function of ketoses may be reduced by the sodium borohydride and a mixture of epimeric products is obtained. D-fructose is the sweetest among all the natural sugars and its reduction gives the mixture of the D-mannitol, and D-glucitol (sorbitol) and they are named after the aldohexose, and they also can be obtained from them by the analogous reduction. The mannitol is also a common carbohydrate found in nature. Generally saying, ketoses are the distinctive isomers of the aldose’s monosaccharides. The facile interconversion of both aldoses and ketoses is possible in the presence of base or acid catalyst because the chemistry of both of the classes is linked. The corresponding epimerization and the interconversion at the alpha sites of the carbon function occurs by the way of the enediol tautomeric intermediate.