Cleansing action is basically a process of physicochemical reactions. These reactions lead to the removal of the soil or the dirt particles from the fabric materials or from any other material. The cleansing action involves the action hemi colloids particles in action. These hemi colloids are basically compounds of hydrosols. Such solutions are formed by the action of the soaps, the synthetic detergents or any other naturally occurring material.
The cleansing action includes the processes of wetting, peptization, emulsification and stabilization of the solid soil particles. These processes take place in a highly dispersed solution of tiny solid particles uniformly distributed in the cleansing solution.
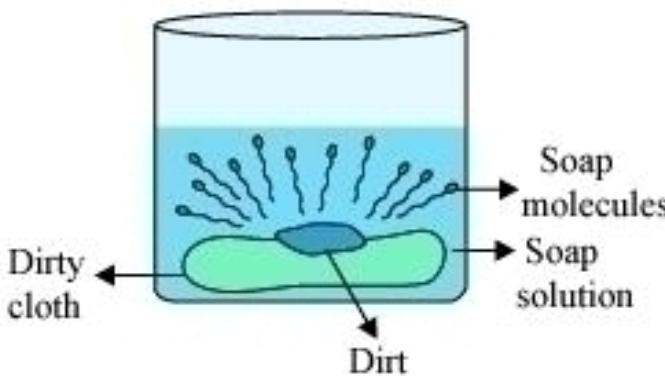
The cleansing action is basically divided into different parts. They are as follows:
Wetting
The wetting process is the foremost and one of the important processes of the cleansing action. Wetting of the soil particles basically requires very less amount of surface tension. The surface tension required for the wetting process is nearly about 30 millinewtonper meter. In the case of the cleansing action, the solid is covered by the oil agents of the cleansing agents. This cover gets dissolved onto the solid particles and eventually reacts with the soil particles. There is adsorption of the cleansing agent on the soil particles.
Emulsification
We know that the surfactants contain a hydrophobic and a hydrophilic part. When the surfactant or soap is added into the water along with the material with dirt certain reactions take place. The hydrophobic part gets attached to dirt and gets bonded to it. Whereas the hydrophilic part faces outward. This leads to the formation of a circular molecular type of structure consisting of dirt and the cleansing agents. This molecule that is formed, is known as a Micelle. The micelles are negatively charged particles as the polar part gets dissolved in the water and the non-polar part gets dissolved into the dirt. Thus, a suspended stable emulsion is formed due to the formation of the micelles in the solution.
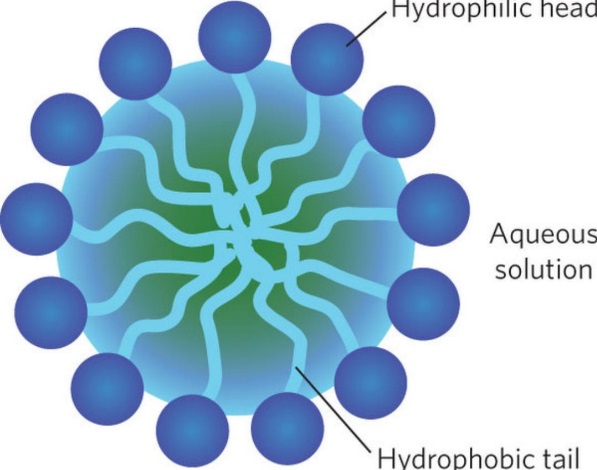
Peptization
The emulsification and the peptization processes go hand in hand, in the cleansing process. The emulsification process majorly focuses on the micelle formation. But, the peptization process the is basically the formation of the stable emulsion solution in the cleansing process. The possibility of forming of stable emulsions arises from the fact that the micelles are negatively charged particles. Also, the micelles are hydrophobic in nature. Hence, the process of adding reagents into the solution, majorly appetising agents to make an emulsion stable is a peptization.
Stabilization
The stabilization process is basically the stabilizing of the emulsions of micelles by the addition of some peptizing agents into the solution.