p-Block Elements: Coordination compounds
Ligands
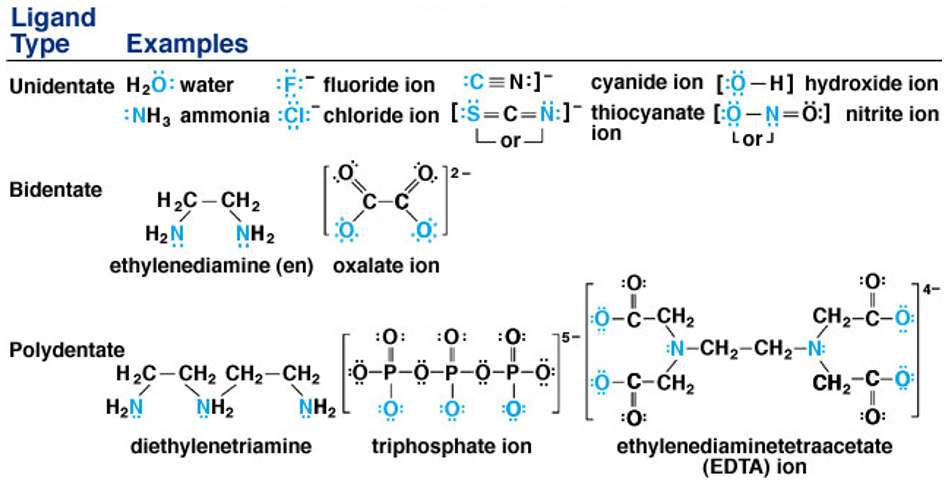
Any species capable of donating a pair of electrons to metal is called a ligand. This ligand is an ion which can either be negatively or positively charged, or a neutral molecule. Examples: Cl– , Br– , SO42- , NH2NH3+, NH3, H2O, NH2CH2CH2NH2 etc.
Ligands can be classified as into three main types: monodentate, bidentate and polydentate
- Monodentate or unidentate ligands-These are ligands which bond to the central metal atom through a single atom, e.g. ammonia (NH3) which bonds to the central metal atom through a nitrogen atom. Other examples are Cl–, CN–, H2O etc. Chelating agents are capable of forming more stable complexes than the complexes that monodentate ligands make.
- Bidentate ligands- These are ligands that connect the central metal atom with other two atoms, e.g. oxalato(C2O42- ), acetylacetonato (acac- ), ethane -1, 2 diamine (en) etc.In these examples, the coordination to the metal atom can take place through the four nitrogen atoms.
- Polydentate ligands- These include ligands that have denticity higher than two. The various types of ligands included are :
- tridentate (denticity of 3), e.g. diethylenetriamine (dien), terpyridine (terpy) etc.In this example, the coordination to the metal atom can take place through the three nitrogen atoms.
- tetradentate (denticity of 4), e.g. triethylenetetramine (triene). In this example, the coordination to the metal atom can take place through the four nitrogen atoms.
- pentadentate ligand (denticity of 5), e.g. ethylenediaminetriacetato. In this example, the coordination to the metal atom can take place through two nitrogen atoms and three oxygen atoms from the acetate group.
- hexadentate ligand (denticity of 6), e.g. ethylenediaminetetraacetato (EDTA4-). In this example, the coordination to the metal atom can take place through the two nitrogen atoms and four oxygen atoms of the acetate group.
In many bidentate to polydentate ligands, the ligand is bonded to the same central metal atom at two or more places due to which a ring structure is formed. A ring structure such as this is formed by a bidentate (or polydentate) ligand that is also known as a chelate. The term chelate, derived from the Greek word chela meaning great claw of the lobster or other crustaceans, is suggested for the clipper like groups which function as two associating units. Chelated complexes have higher stability in comparison to other ordinary complexes. Ethylenediaminetetraacetic acid (H4EDTA) is an important chelating ligand. It binds the metal atom through two nitrogen and four oxygen atoms and hence acts as hexadentate ligands and forms five rings.
Ligands that bind to metal ions through two atoms of different elements present in it are known as ambidentate ligands. Example of such ligands is NO2– and SCN– ions. NO2– ion can form coordination compound by either binding through nitrogen or through oxygen to a central metal. Similarly, SCN– ion can coordinate through small sized atoms such as sulphur or nitrogen atom.