d and f Block Elements
Transition metals: colour
Transition metals have a specific tendency to form complex ions with water in their aqueous state. In compound state due to the surrounding ligands, the d-orbitals of transition elements are not degenerate. However, they tend to split into two groups of different energy to promote electrons from one group to another group. This corresponds to a small amount of energy transfer by absorbing and so light is absorbed in the visible region. For example, ZnSO4 and TiO2are white in colour, for example. In these compounds, the electrons are another sub-level within d orbital making the energy exchange higher than the visible band thereby absorbing UV light. Hence, the origin of the colour is electronic transition involving d electrons that corresponds with the energy band in the visible wavelength. Colour in transition-series metal compounds is generally due to electronic transitions of two principal types: charge-transfer transitions and d-d transitions.
Ligand to metal charge transfer (LMCT) allows an electron to jump from a predominantly ligand orbital to a metal orbital when the metal has acquired a high value of oxidation state. The colour of chromate, dichromate, and permanganate complex ions is due to the occurrence of LMCT transitions.
In a d-d transition, an electron in the complex ion jumps from one subshell in the d orbital to another where the energy level of each jump is variant as the orbital splits.
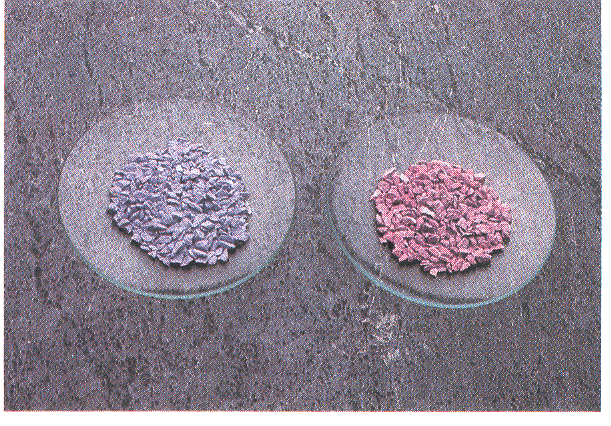
Fig : In anhydrous form CoCl2 is blue but in the hydrated form CoCl2.6H2O is pink
Most of the transition metal compounds (ionic as well as covalent) are coloured both in solid state & in an aqueous state. Generally, the elements/ions having unpaired electrons produce the coloured compound. The colour of light absorbed by the complexed ion is related to electronic energy changes in the structure of the complex.
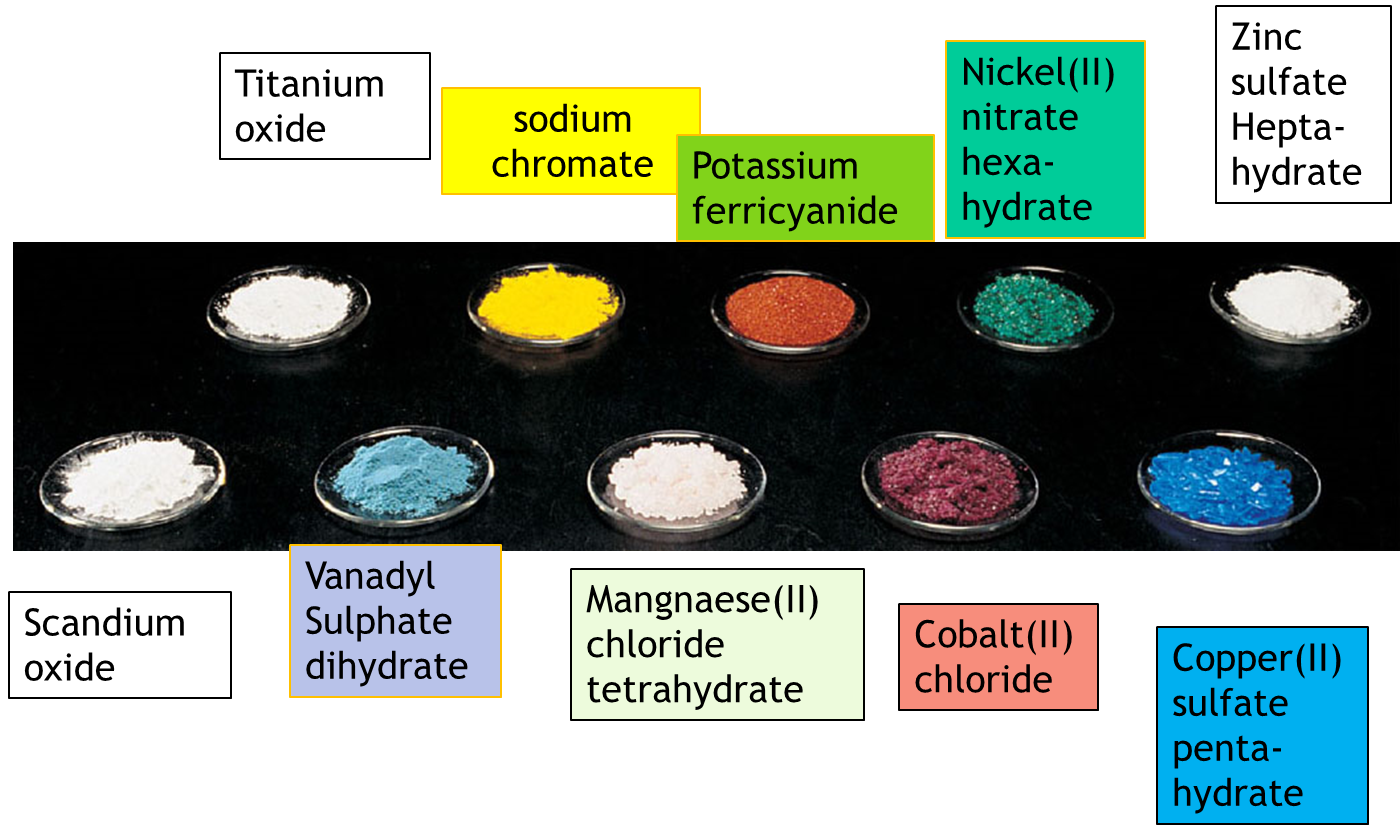
Zn(II) compounds are typically colourless unless the ligands in the complex have a chromophore (a group of atoms responsible for the absorption of electromagnetic radiation) which can absorb in the visible region. Colours of aqueous solutions of compounds containing vanadium in different oxidations states (V, IV, III and II) are shown in the figure below.
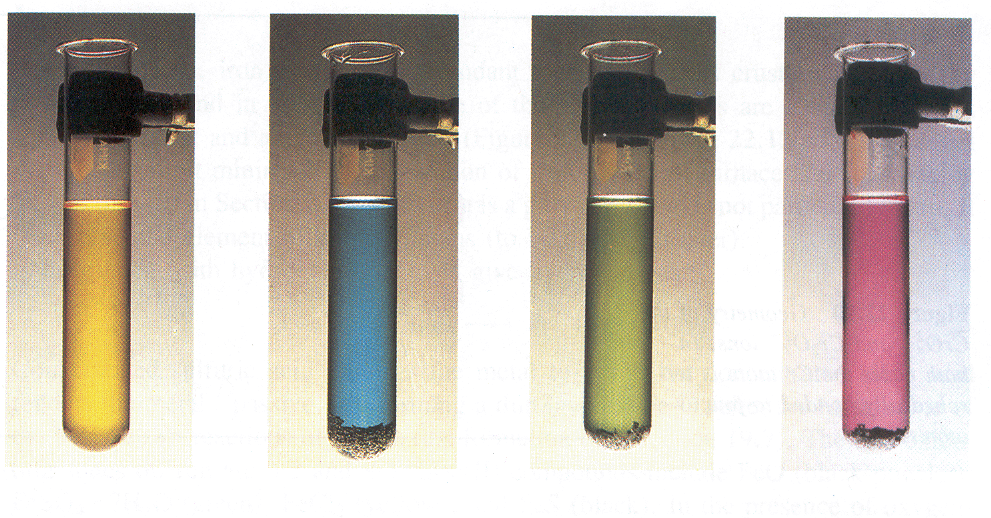
For an explanation of the colour transition metals ions in water solution existing in complex form, we need to consider the bonding in complex ions of transition metals.
Fig : Relation between absorbed and observed colour
The electrical field of neighbouring ions on the energies of the valence orbitals of an ion in a crystal. A substance appears coloured because it absorbs light at one or more wavelengths in the visible part of the electromagnetic spectrum (400 to 700 nm) and reflects or transmits the others. Each wavelength of light in this region appears as a different colour. A combination of all colours appears white, the absence of light waves appears black.
The colours of complex ions are due to electronic transitions between the split d sublevel orbitals. The wavelength of maximum absorbance is utilized to determine the size of the energy gap between the split ofd sublevel orbitals. Hydrated cupric ion is blue because each ion absorbs a photon a wavelength of about 600 nm (orange light), the transmitted light appears blue to our eyes.
Ephoton = hυ = hc/λ = Δ