d and f Block Elements
Magnetic properties
Every spinning electron in an atom or molecule can behave like a tiny magnet. Electrons that are positioned in opposite spins have an opposing orientation to create no net magnetic effect. Therefore, elements and ions with paired electrons and completely filled orbitals do not show magnetic properties. Only if these elements or ions have unpaired electrons, they will show magnetism.
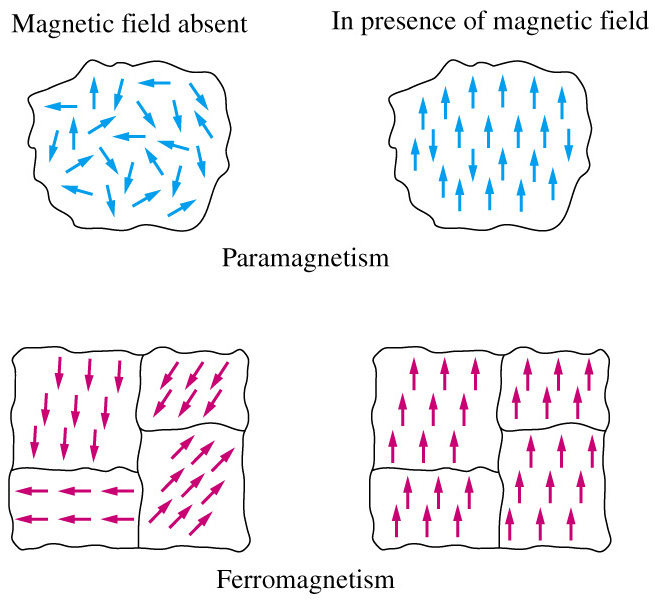
Diamagnetism – materials that contain paired electrons show weak opposition to an applied magnetic field and are called diamagnetic.
Paramagnetism – only occurs with substances with unpaired electrons, the magnetism is proportional to the applied field and in the same direction
Ferromagnetism – only occurs with the long-range ordering of the unpaired electrons, magnetism can be greater than the applied field.
Most of the transition elements and their compounds show paramagnetism due to the presence of unpaired electrons with the magnetic moment and multiple oxidation states.
Hund’s rule states that electrons occupy orbitals of the same energy one by one. This means that when all lower energy orbitals are half-filled:
- The next electron can enter a half-filled orbital and pair up by overcoming repulsive pairing energy, (Epairing).
- The next electron enters an empty higher energy orbital by overcomingΔ.
- Therefore, the number of unpaired e– will depend on the relative sizes of Epairing and Δ.
Consider the example below:
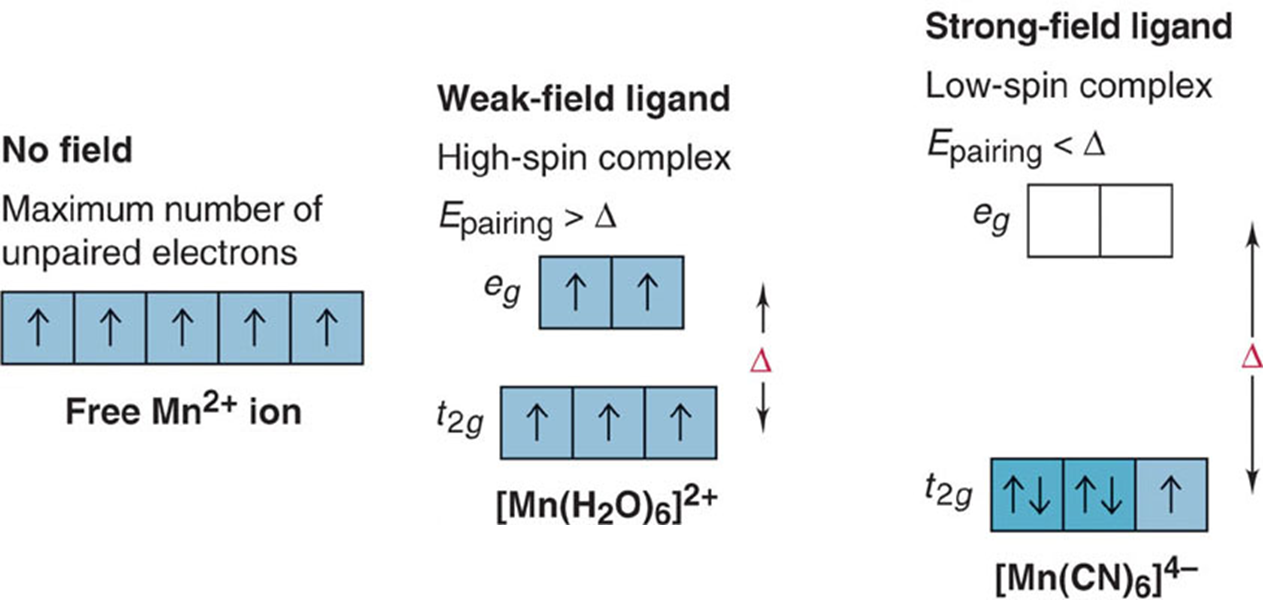
Fig : High-spin and low-spin octahedral complex ions of Mn2+.
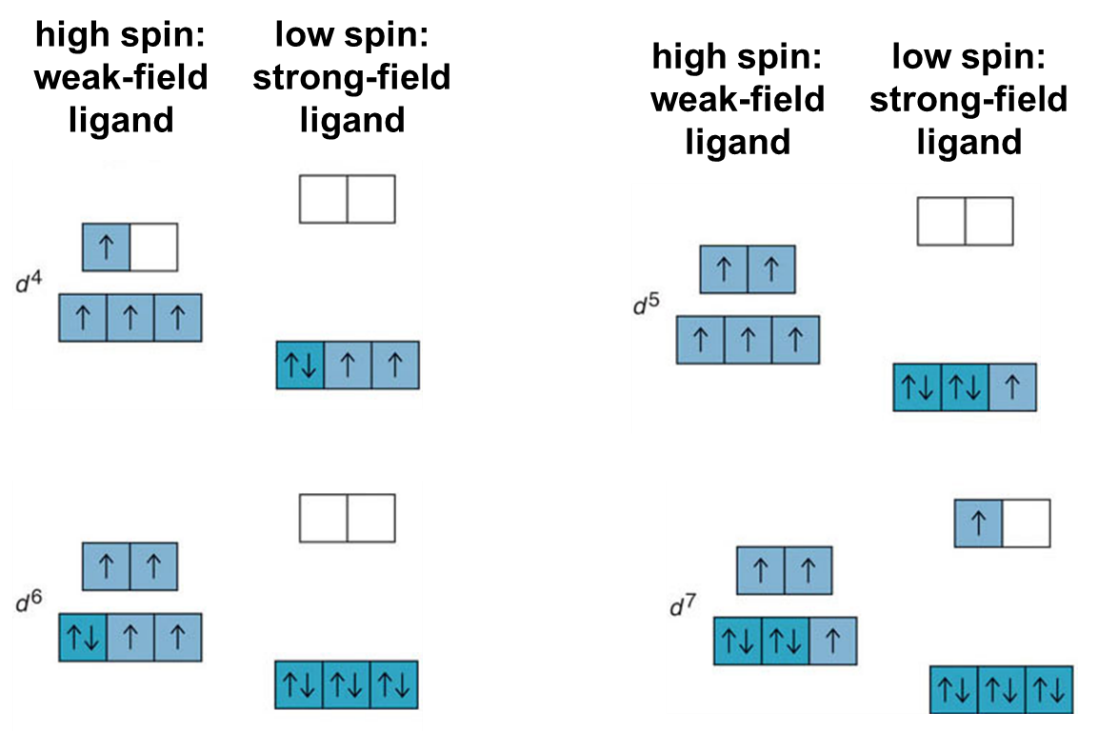
Fig : Orbital occupancy for high-spin and low-spin octahedral complexes of d4 through d7 metal ions.
The magnetic moment of transition element, its compound or ion is given by:
μs = √n(n+2) BM
Here ‘n’ stands for is the number of unpaired electrons. In this equation it is assumed that there is no contribution from the orbital magnetic moment).
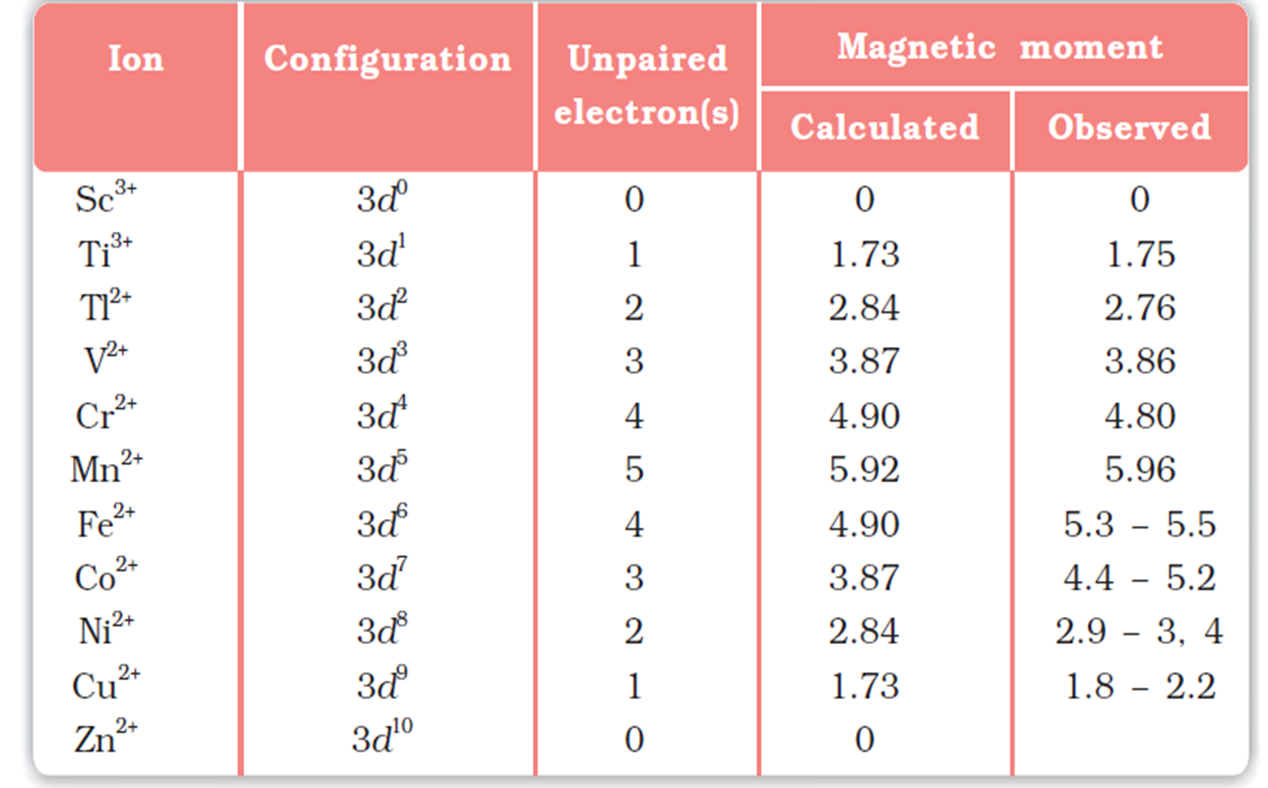
In the first transition element series, the paramagnetism first increases and then decreases. It increases with the number of unpaired electrons so generally increases from left to right across the Periodic Table until Chromium and then it decreases. Zinc is diamagnetic because it has no unpaired electrons. The maximum paramagnetism is seen in the middle of the series where elements have multiple oxidation states. Transition metal ions with a d0 or d10 configuration are also colourless and diamagnetic.
Iron, cobalt and nickel are together called the iron triads and these metals are ferromagnetic. The unpaired electrons in the d orbitals collect in large numbers of atoms and line up with parallel spins in regions called domains. These domains become ordered when they are exposed to an external magnetic field. The magnetism can remain after the magnetic field is removed. Industrial magnets are made from an alloy of nickel, cobalt, and aluminum. Nickel is used in batteries along with cadmium. Iron is a necessary part of hemoglobin, the substance that transports oxygen in the blood.
Both Zn atoms and Zn2+ ions are diamagnetic: Zn atoms [Ar]4s23d10 and Zn2+ ions [Ar]4s03d10
Ag forms both Ag+ ions and, rarely, Ag2+ :Ag atoms [Kr]5s14d10 are paramagnetic, Ag+ ions [Kr]4d10 are diamagnetic and Ag2+ ions [Kr]4d9 are paramagnetic.