In synthetic organic chemistry, diazonium salts are widely used compounds. At first, diazonium salts were used for the production of water fast dyed fabrics and the reaction involves the immersing of fabrics to an aqueous solution of the diazonium compound. Then it was immersed in a solution of the coupler, which is the ring which is electron-rich and undergoes the electrophilic substitution reactions. Diazonium salts have major applications in the field of pigment and dye industry and they are used to make the dyed fabrics.
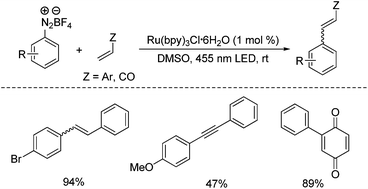
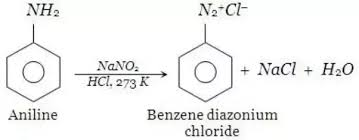
Nowadays, diazonium salts are widely used to make the azo dyes, so they have significant importance in the industry, and synthetic organic chemistry. The preparation of azo dyes is generally done in two-step reactions, In the first step, the aromatic diazonium ion is synthesized from the derivative of aniline. In the next step coupling of diazonium salt is done with an aromatic compound. The different shades of azo dyes include orange, brown, blue, yellow, and red.
These azo pigments are important in various industries such as paints, rubber, and plastics. They are preferred to the light fastness and their excellent coloring pigments. Many of the azo-pigments are nontoxic and only a few of them are carcinogenic and mutagenic. Even, many classes of the azo dyes are known that are produced by the reactions of diazonium salts, such as substantive dyes, reactive dyes, and metal complex dyes. In the cellulose-based textiles, such as cotton, substantive dyes are used. These dyes are bound to the textile by the non-electrostatic forces. Some of the azo compounds such as the methyl orange are being used as the acid-base indicators. Most of the proteins are cationic, so the dyeing of wool and leather corresponds to the ion exchange reaction. The anionic dyes adhere to the articles by the electrostatic forces. Quaternary ammonium centers are present in the cationic azo dyes. Azo pigments are also produced from the diazonium salts and their chemical structure is similar to the azo dyes.
In the synthesis of organic compounds, diazonium compounds are standard reagents, especially the aryl derivatives. Substituted aromatic compounds cannot be obtained by direct substitution in the benzene and for these compounds, the replacement of diazo compounds is used in the diazonium salts. For introducing the fluoride, bromide, chloride, iodide, hydroxyl and -CN groups to the aromatic ring, diazonium salts are used as intermediates.