The amines are formed as the derivatives of the ammonia compound. The derivatives are formed by the replacement of the hydrogen atoms from the ammonia. The amines have an alkyl or aryl group that replace the hydrogen atoms. The amines are categorized into the primary, secondary, tertiary and cyclic amines depending upon the number of alkyl or aryl groups attached to the nitrogen atom. The amines serve many uses in our day to day lives. The amines are used for making medicines.
Physical properties of amines
- The amines are organic compounds with alkyl or aryl groups attached to the nitrogen atom with one lone pair of electrons on the nitrogen.
- In the aliphatic amines, the lower amines exist in the gaseous state. Whereas, the higher aliphatic amines exist in the liquid state. In the aryl amines, the lower amines exist in the state of liquid. But, in the higher order of aryl amines, the amines have solid state of nature.
- The smell exhibited by the methyl amine and the ethyl amine is sort of ammonia smell. Whereas, the higher order amines have some fishy type of smell.
- Most of the aromatic amine compounds in the industry are colourless in their pure. However, the exhibit after standing for a long time due to oxidation in the air. Aromatic amines are usually toxic in nature and may permit easily through the skin. Hence, proper safety precautions should be taken while dealing with aromatic amines.
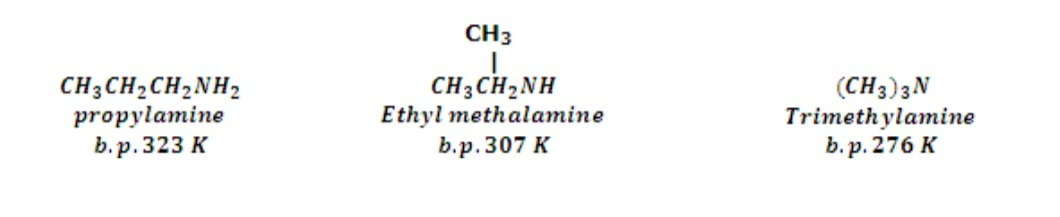
- The primary and the secondary amines have a higher boiling point than the non polar molecules of same molecular masses. The difference in the boiling points is due to the fact that the amines are capable of making intermolecular Hydrogen bonding between each other. But, the tertiary amines are incapable of making H bonds hence have comparatively lower boiling points than other.
Chemical properties of amines
The amines have different chemical properties. They are:
- They amines easily react with aqueous mineral acids and get converted into their salts. They react with aqueous hydroxides and get liberated from their salts. Hence, the amines are more basic than the water and less basic than hydroxide ions.
- The amines react with alkyl halides to form the amines of a higher order. The nitrogen atom with the ligands acts as a nucleophile and thus attacks the alkyl halide by the nucleophilic attack reaction.
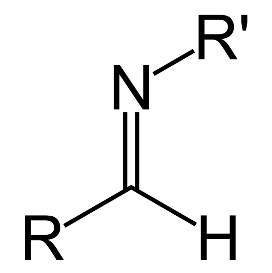
- Primary amines react with the carbonyl group of the ketones and the aldehydes to formcarbinoamines. The carbinoamines are further dehydrated to the immines which are also known as Schiff’s base. The type of reaction here is a nucleophilic reaction.