The Standard Electrode Potential is a measure of potential for the equilibrium state. There’s a difference in potential which is the potential difference between the electrodes in addition to the electrolyte which is known as electrode’s potential. When there is concentration present in unity of all species indulged in semi-cell, then the electrode potential is referred to as cell’s standard electrode potential.
Under standard conditions, the standard electrode potential takes place when enclosed in the electrochemical cell with the temperature = 298 K, concentration = 6 M, pressure = 1 atm. The symbol ‘Eocell’ depicts the standard electrode potential of the cell.
Example of Standard Electrode Potential
The Standard Electrode Potential calculation of zinc electrode is done bu using the standard hydrogen electrode as described below:
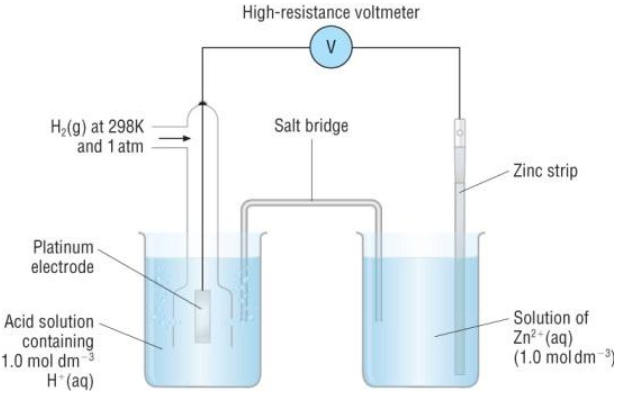
Note that this potential is being measured following standard conditions where the pressure is 1 atm, and the temperature is 298K, and the electrolytes’ concentration is 1M.
The electrochemical cell’s basis like the galvanic cell, is continually the redox reaction that can be fragmented into 2 half-reactions namely, reduction process at cathode (electron’s gain), and oxidation process at anode (electron’s loss). The generation of electricity is because of an electric potential difference present in between the 2 electrodes.
There’s generation of potential difference as a consequence of difference between the individual potentials of 2 metal electrodes w.r.t electrolyte. The reversible electrode refers to the electrode which is obligated to its potential to alterations of reversible behaviour, on contrary to electrodes which is used in electroplating that are destroyed at the time of their use. It’s the measurement of the reducing power of compound or any element.
There is no easy way to measure electrolyte/electrode potentials precisely in isolation. However, the complete potential of the cell can be measured.
The electric potential changes with concentration, pressure, and temperature. Since half-reactions’ oxidation potential is negative in a redox reaction, having the reduction potential, it’s then sufficient enough to calculate one or the other potentials. Thus, standard electrode potential is usually expressed as standard reduction potential.
The half-reactions that actually occur in the cell and their corresponding electrode potentials are as follows:
cathode:
2H(aq)^+ +2e^− →H2 (g) E°cathode = 0V
Anode:
Zn(s) →Zn2(aq)^+ + 2e^− E°anode=−0.76V
Overall:
Zn(s)+2H(aq)^+→Zn(aq)2^+ + H2(g)
E°cell = E°cathode−E°anode = 0.76V
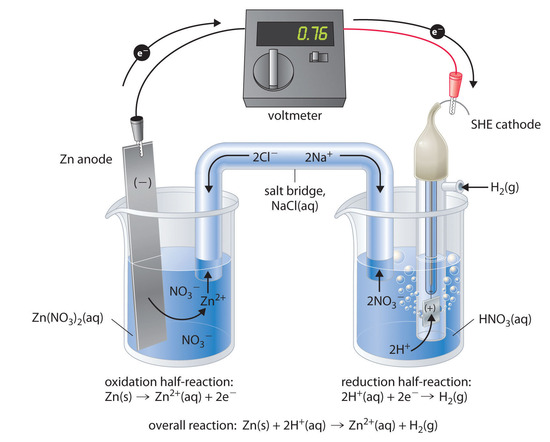
Even though, the reaction taking place at anode is the oxidation, by the usual way, its E° value in tabulated form is stated as the reduction potential.
The half-reaction’s potential is measured in contrast to the SHE, following standard conditions is known as the standard electrode potential for that specific half-reaction.
In this eg., the standard reduction potential as mentioned-above, for Zn2+(aq) + 2e− → Zn(s) is −0.76 V, which signifies that the standard electrode potential for the reaction taking place at anode, is the oxidation of Zn to Zn2+, generally defined as Zn/Zn2+ couple, or Zn/Zn2+ redox couple, is −(−0.76 V) = 0.76 V.
Therefore, we should subtract the factor E°anode from the E°cathode to attain E°cell: 0 − (−0.76 V) = 0.76 V.