d and f Block Elements
Actinides: Electronic configuration
The elements with occupied 5f- orbitals of (n-2)th are known as 5f-block elements, actinides. There are only thirteen elements from Th90 (5f0 6d2 7s2 ) to No102 (5f14 6d0 7s2 ) should be the members of the actinide series. However, all the fifteen elements from Ac89 (5f0 6d1 7s2 ) to Lw103 (5f14 6d1 7s2 ) are considered as the members of actinide series, since all these fifteen elements have same physical and chemical properties. In fact, actinium is the prototype of actinides as lanthanum is the prototype of lanthanides. The forth transition series (inner) or 4f series is incomplete. It has 15 elements: Actinium (Ac) to element 104 through element 109: 6d subshell is filling, If elements 110 and 111 are found then this will complete this series. From neptunium onwards, the elements are man-made and they are known as transuranic elements.
The general electronic configuration of actinoids is [Rn] 5f0-14 6d0-1 7s2. The difference in energy of 5f and 6d is not significant so it is difficult to predict whether the electrons have entered 5f or 6d orbital.
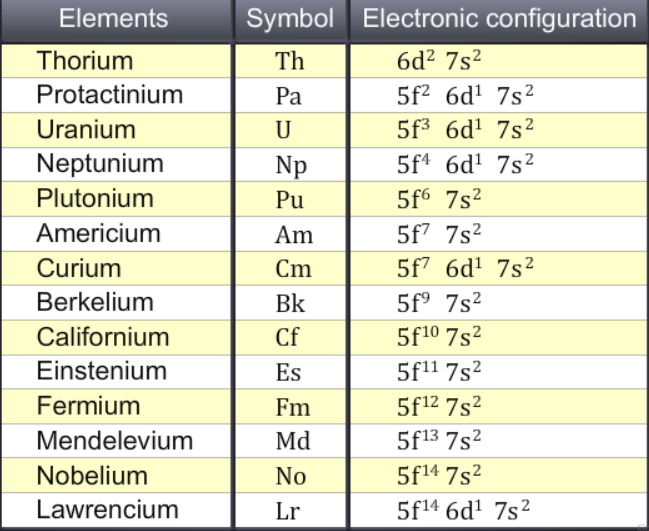
From the above valence shell configurations of the actinide elements, the electronic configuration of actinium (Z = 89) which is followed by fourteen actinides is [Rn]5f0 6d1 7s2, the last electron entering the 6d-subshell. In the next element, Th, the first member of the actinide series, the additional electron must enter 5f-subshell and the filling of 5f-subshell must continue progressively till the last element, Lr. Thus, 6d-subshell in all the elements must remain singly filled thereby giving the expected valence shell configuration of 5f1-146d1 7s2 for these elements. Since, the energies of 6d- and 5f- subshells are almost the same and the atomic spectra of the elements are very complex, it is difficult to identify the orbital in terms of quantum numbers as well as to write down the configuration. For chemical behaviour, the valence shell electronic configuration of the elements is of great importance and the competition between 5fn 6d0 7s2 and 5fn-16d1 7s2 is of interest. It has been observed that the electronic configuration of actinides does not follow the simple pattern as is observed for the lanthanides. For the first four actinide elements: Th, Pa U and Np, due to almost equal energies of 5f and 6d, the electrons may occupy the 5f or 6d subshells or sometimes both. From Pu (Z=94) onwards, the 6d1 electron gets shifted to 5f-subshell except for Cm (Z=96) and Lr (Z=103) in which 6d1 electron does not shift to 5f due to stable 5f7 and 5f14 configurations.
It is clear that Th does not have any f-electron though this element belongs to 5f-series (i.e.,actinides). For Pa, U, Np, Cm and Lr, both the expected and observed (actual)configurations are the same. For the rest of the actinides, 6d-subshell does not contain any d-electron.