d and f Block Elements
Ionic radii
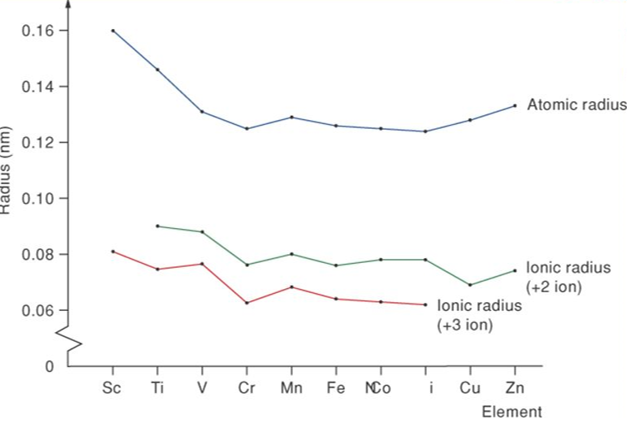
A great degree of variation is seen in the atomic radii across each transition series. The atomic radii of the d-block elements within a given series decrease with increase in the atomic number. This is due to the increase in the nuclear charge that attracts the electron cloud inwards resulting in a decrease in size. However, a uniform decrease in atomic radius is not observed across the first period in transition metals. The decrease in atomic radii is small compared to the S and P block elements. This is due to the screening effect caused by the electrons of the (n-1)d subshell on the outermost shell. As a result, the nucleus cannot pull the outermost electrons. Thus, the atomic radius does not alter much from Cr to Cu.
The atomic radius increases on descending the group. In a given series, the atomic radius decreases to a minimum for the group VIII elements and then it increases towards the end of the series. This increase in radius towards the end of the series is due to the force of repulsion among the added electrons. A close similarity is observed in the radii of the elements of the second and third transition series due to the filling of 4f subshells.
A cation forms by the loss of one or more electrons; This leads to a decrease in atomic radius because the removal of the highest-energy-level electrons causes a smaller electron cloud. An anion forms by the addition of one or more electrons; which always causes an increase in electron cloud size. Transition metals form cations by an initial loss of ns e-’s, even though ns orbital is lower in energy than (n–1)d subshell in the neutral atom. All transition-metal cations possess dn valence e- configurations for 2+ ions of 1st row.
The ionic radius is similar to the pattern of atomic radii. Thus, for ions of a given charge, the radius decreases slowly with increase in atomic number. The order in which electrons are removed from all atoms of the d-block and f-block elements is exactly the reverse of the order given by the electron-configuration notation. In other words, electrons in the highest occupied sublevel are always removed first. For the d-block elements, this means that although newly added electrons that occupy the d sublevels are not removed first; the first electrons to be removed are those in the outermost s sublevels.
For example, iron, Fe, has the electron configuration [Ar]3d64s 2. To become an ion, Fe loses a 4s electron first to form Fe+ ([Ar] 3d 6 4s1 ). Fe+ ion loses the second 4s electron to produce Fe2+ion: ([Ar] 3d 6 ). Fe2+ion loses a 3d electron to produce an ion of +3 state: Fe3+ ([Ar] 3d 5 ). Most d-block elements have +2 oxidation state and they can stably form2+ ions in compounds while iron and chromium can form 3+ ions. Cu forms 1+ and 2+ ions, and Ag usually prefers only 1+ ions. As expected, the cations have smaller radii than the atoms do. Comparing 2+ ions across the periods follows the decrease in size that parallels the decrease in atomic radii.