d and f Block Elements
Preparation and properties of K2Cr2O7 and KMnO4
A complex ion is formed when a central ion is surrounded by molecules or ions which possess a lone pair of electrons. The relatively high charge and small size of the transition metal allow them to attract the ligand’s lone pair of electrons. These “ligands” are attached via a coordinate bond. A coordinate bond uses a lone pair of electrons to form a covalent bond.A ligand is a species that uses a lone pair of electrons to form a coordinate bond with a metal ion.
Potassium dichromate K2Cr2O7
The chromate ion has a tetrahedral structure whereas the dichromate ion consists of two tetrahedra that share one corner with Cr–O–Cr bond angle of 126°. Potassium dichromate is used as an oxidizing agent in volumetric analysis. In industrial-scale, it is used in mordant dyes, leather industry, photography, cleaning glassware etc.
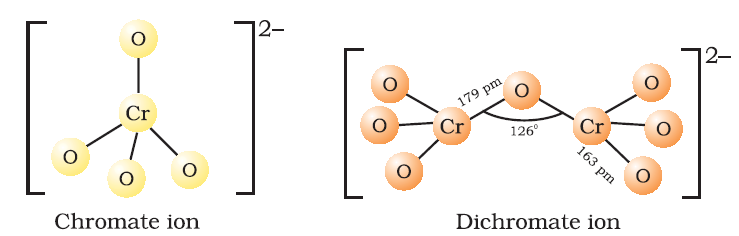
Dichromates are generally prepared from chromate, which in turn are obtained by reacting chromite ore (FeCr2O4) with sodium or potassium carbonate in free access of air.
4 FeCr2O4 + 8 Na2CO3 + 7 O2 → 8 Na2CrO4 + 2 Fe2O3 + 8 CO2
2Na2CrO4 + 2 H+ → Na2Cr2O7 + 2 Na+ + H2O
Na2Cr2O7 + 2 KCl → K2Cr2O7 + 2 NaCl
In acidic solution,its oxidising action can be shown as follows:
Cr2O72– + 14H+ + 6e– → 2Cr3+ + 7H2O ;
e.g.
6 I–→ 3I2 + 6 e–
3 H2S → 6H+ + 3S + 6e–
3 Sn2+ → 3Sn4+ + 6 e–
6 Fe2+ → 6Fe3+ + 6 e-
Dichromate ion is orange acidic solution (pH<7) and it turns yellow in a basic solution due to interconversion of dichromate ion to chromate ion. Following reactions take place:‐
2 Cr042‐ (yellow) +2 H+ → Cr2O72‐ (orange) + H2O
Cr2O72‐ (orange) +2 OH‐ → 2 Cr042‐ (yellow) + H2O.
Potassium Permanganate, KMnO4
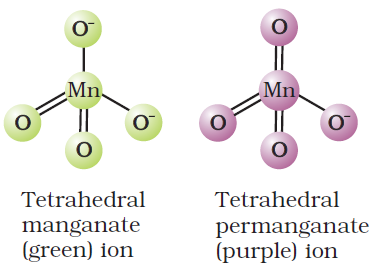
Potassium permanganate is a tetrahedral complex compound. In laboratory-scale, it is used in preparing chlorine gas and other oxidization reactions. In industrial-scale it is used in making disinfectants, Baeyer’s reagent and other oxidizing purposes.
It is prepared by mixing MnO2 with an alkali metal hydroxideand an oxidising agent(O2 or KNO3) . The reaction produces dark green K2MnO4 which disproportionates in a neutral or acidic solution to give permanganate.
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
3MnO42– + 4H+ → 2MnO4–+ MnO2 + 2H2O
In the laboratory, manganese (II) ion salt is oxidised byperoxodisulphate to permanganate.
2Mn2+ + 5S2O82– + 8H2O → 2MnO4– + 10SO42– + 16H+
This compound has a strong oxidising agent in acidic as well as in neutral& basic medium :
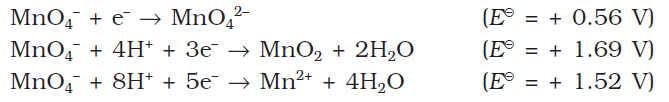
In acidic solutions:
Iodine is liberated from potassium iodide:
Fe2+ ion (gree) is converted to Fe3+ (yellow):
Oxalate ion or oxalic acid is oxidized at 333 K:
Hydrogen sulphide is oxidized and Sulphur is precipitated:
Sulphurous acid or sulphite is oxidized to form a sulphate or sulphuric acid:
Nitrite is oxidized to form nitrate:
In neutral or faintly alkaline solutions:
Iodide is oxidized to iodate:
Thiosulphate is oxidized almost quantitatively to sulphate: