The Fuel cells produce the electricity via the chemical reaction, but the condition is there’s no combustion. It does convert the Hydrogen plus the oxygen into the water, and during the process, it also creates the electricity. It refers to a device which there’s the conversion of electrochemical energy which produces water, electricity, and heat.
Here, in the below diagram is shown the working of the fuel cell
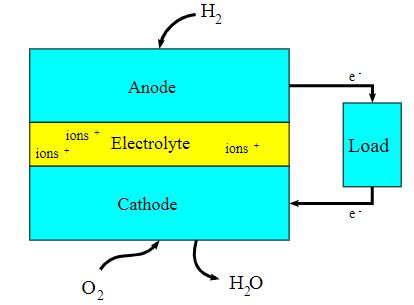
The reactions taking place here are:
The Reaction In Cathode: O2 + 2H2O + 4e– → 4OH–
The Reaction in Anode: 2H2 + 4OH– → 4H2O + 4e–
The Net or Total Cell Reaction: 2H2 + O2 → 2H2O
Efficiency of Fuel Cell:
Fuel cell’s efficiency is illustrated as electricity’s generation which is usually estimates around 70%. On the other hand, 70% of the thermal energy generated power plants consist of efficiency belonging to 40%.
This noticeable difference in context of efficiency is due to electric current generation in the field of thermal power station which includes water conversion into vapor state or steam, and the use of this steam is applied for rotating a turbine. However, fuel cells provides you the centre stage for direct conversion of the chemical energy into the electrical energy.
The Fuel Cells work very much like batteries, except the fact that they do not need electricity recharging. The battery does store the whole of its chemicals inside plus it converts the chemical form into electricity form. Once the particular chemicals are released or are exhausted, the battery doesn’t work at all as it dies.
On the flip side, a fuel cell acknowledges the chemicals which it manages and uses from the exterior or the outside world. Thus, it would not be exhausted or run out. Fuel cells have the ability to generate power nearly indefinitely considering that they are having that fuel for the application or to use.
The reactions which are electricity generators, they have their working taking place at electrodes. Each of the fuel cells consists of 2 electrodes, one is the positive one which is known as Anode and the other one is the negative one which is the cathode. These electrodes are being separated by the electrolyte barrier.
Fuel goes towards the anode, while the oxygen (or just the air) goes towards the cathode. When these two chemicals collide with the electrolyte barrier, it results in some sort of reaction, and divides their electrons, and thus results in the creation of an electric current. The chemical catalyst here accelerates the reactions.
Advantages of fuel cells
When the power is required by you, the fuel cells can be the perfect solution for your problems.
There are so many types of fuel cells like:
- Phosphoric Acid Fuel Cell
- PEM or Polymer Electrolyte Membrane Fuel Cell
- Alkaline Fuel Cell
- Molten Carbonate Fuel Cell
- Solid Acid Fuel Cell
- Solid Oxide Fuel Cell
A few of the applications of Fuel Cells:
- Fuel Cell makes use of FCEVs and therefore, know for their eco-friendly nature.
- A very good health advantage is that Hydrogen fuel cells generate or in a way produces water and heat only
- The energy efficiency is 2 to 3 times more as compared to the combustion engines
- The fuel cells can be effortlessly combined with alternative energy technologies such as solar panels, wind turbines, batteries, and supercapacitors.
- Fuel cells are versatile in nature as well as they are scalable.