Point defects
Point defects describes the imperfections in solids alongside point defects types. The formation of crystalline solids takes place by connecting great numbers of crystals, smaller in size. After the crystallization process, various defects types are observed in the crystal.
Point defects are calculated when the process of crystallization takes place at a pretty fast rate enough. Mainly, these defects occurs because of deviation found in the arrangement of the constituent particles. The perfect arrangement of solids in the crystalline solid is distorted or disfigured surrounding the atom or point, which is know as the “Point Defect”.
Imperfections or defects in solids, crystalline in nature can be categorized into 4 groups, which includes:
- Volume defects
- Point defects
- Line defects
- Surface defects
As what recorded, the point defects of crystal were initially considered in ionic crystals, but not in metal crystals which were very simple.
Mainly, 3 types of point defects are there:
- Stoichiometric defect
- Frenkel defect
- Schottky defect
Stoichiometric Defect:
Stoichiometric point defect represents the ratio of positive ions with negative ions (Stoichiometric). In this, solid’s electrical neutrality is not interrupted. Occasionally, it’s also called thermodynamic or intrinsic defects.
Basically, there are two types of Stoichiometric Defect:
-
- Vacancy defect: When the atoms do not exist at their lattice sites, then there’s vacant lattice site and it does create the “vacancy defect.” Because of this, the substance’s density reduces.
- Interstitial defect: Interstitial defect is a defect in which a molecule or an atom occupies intermolecular spaces in the crystal. In this very defect, substance’s density increases.
Mainly, the non-ionic compound indicates interstitial and vacancy defects; whereas the ionic compound depicts the same in Schottky and Frenkel defect.
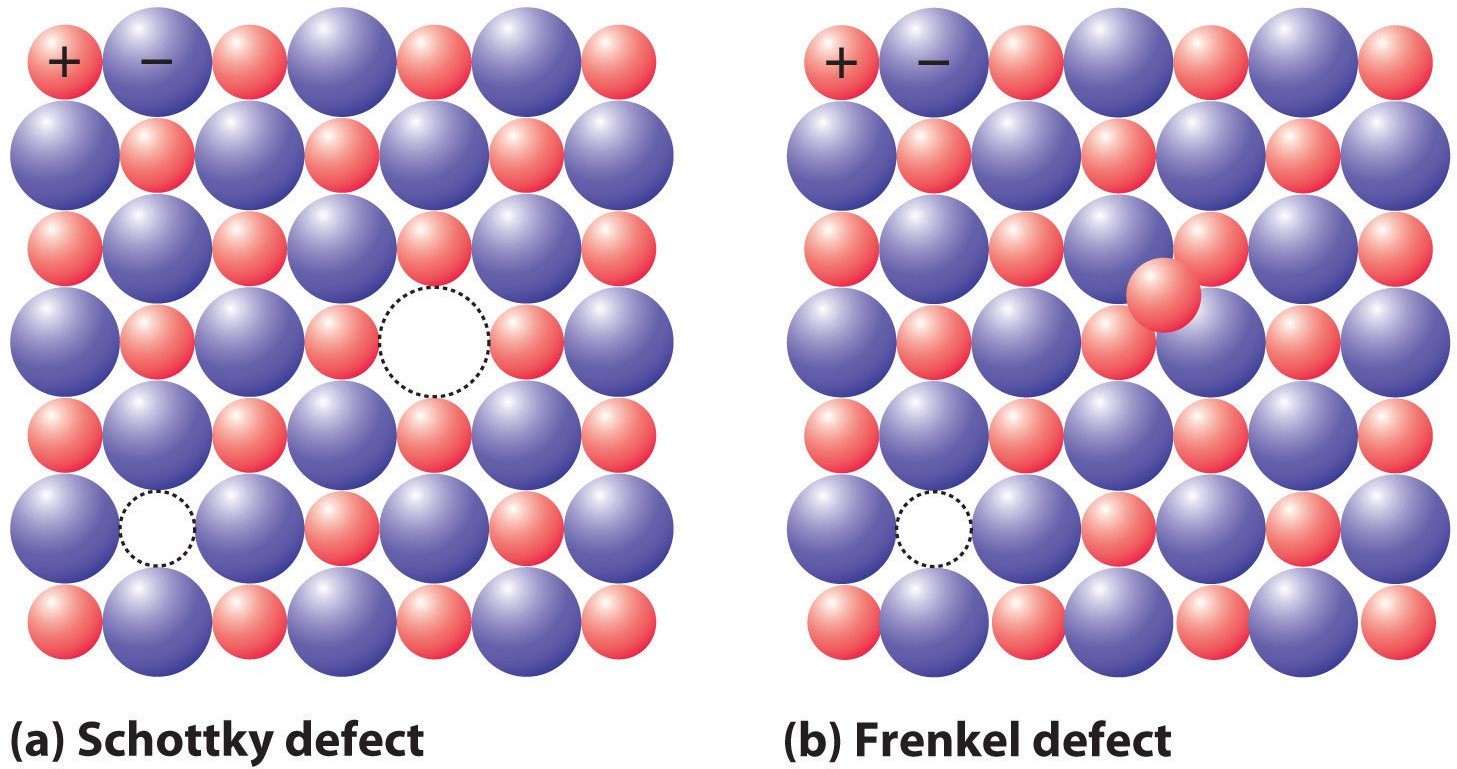
Frenkel Defect
Generally, in ionic solids, cations, the smaller ion exits from its location and occupies the intermolecular space. In this condition, a “vacancy defect” is build on its original place and “interstitial defects” are seen in its new location.
-
- Also called dislocation defect.
- Substance’s density is not changed
- Density is unchanged when there’s a big difference in cations’ and anions’ size
- Eg: AgCl and ZnS
Schottky Defect
-
- Schottky vacancy defects are found in Ionic Solids. But when you have ionic compounds, there’s a need to maintain compound’s electrical neutrality. So, there’s the same number of positively charged cations and negatively charged anions which will disappear from the compounds.
- Substance’s density gets reduced
- Anions and Cations’ size are almost equal or the same
- Impurity Defect: This defect can be easily understood by the example of molten NaCl being crystallized along with compound of SrCl2. In this, Sr2+ ions replace 2 Na+ ions and reside in the place of single Na+. In this manner, the lattice location of 1 Na+ is empty and it generates the impurity defect.
- Non-Stoichiometric Defect: In Non-Stoichiometric Defect, the ratio of anions and cations is disturbed because of either the addition or removal of ions.
Types of Non-Stoichiometric Defect:
- Metal Deficiency Defect
- Metal Excess Defect
- There are two types of metal excess defect:
- Metal excess defect because of anionic vacancies.
- Metal excess defect because of additional cations presence at interstitial sites