Introduction
Exchange of gases in simple organisms occurs directly with the environment, unlike in complex animals where the gaseous exchange occurs the atmosphere and the blood.
The need for gas exchange.
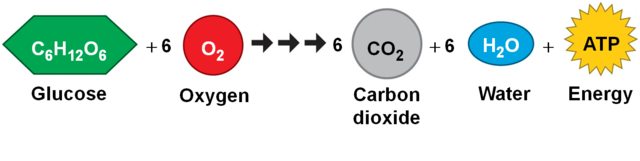
The process by which cells convert food into energy for use is called cellular respiration. Aerobic respiration produces larger amounts of energy but requires oxygen, producing carbon dioxide as a waste. To maintain cellular energy, efficient gas exchange is required to provide oxygen and remove carbon dioxide.
Gaseous exchange.
Gaseous exchange occurs between alveoli and blood in the capillaries that nourish the lungs.
Adaptation of alveoli.
- Number: there are 350 million alveoli per lung, which equates to 700 million in a human being.
- Small size: the small size provides a larger surface area to volume ratio than large structures.
The alveoli walls and those of capillaries are just one cell thick, providing a short diffusion path. The alveoli walls are lined with a thin film of moisture where gases dissolve, creating an even shorter diffusion path. Steep concentration gradients are set to ensure diffusion of gases to accommodate the constant intake and removal of unwanted gases.
Factors affecting the diffusion rate.
- Distance- the diffusion path is small, thus creating a shorter diffusion path for faster diffusion since the particles do not have to travel over a long distance.
- Surface area- a large surface area provides a higher number of particles that travel in a given time, thus faster rate.
- Concentration gradient- diffusion is faster in a scenario where there is a high concentration of particles at the origin compared to the destination.
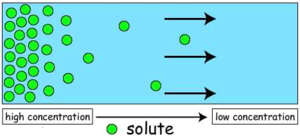
Alveoli are adapted to have a larger surface area, short diffusion distance and a big concentration gradient. All these enable alveoli to have a rapid diffusion rate as a survival mechanism for organisms since there is constant need to obtain enough oxygen, amino acids and sugar used in metabolism and removal of waste substances such as urea in the shortest time possible.
Fick’s Law.
Fick’s law states that “the rate of diffusion is proportional to both concentration difference and surface area and inversely proportional to the thickness of the membrane.”
This law is written as:
![]()
It, therefore, describes the relationship between the diffusion rate and the three factors affecting diffusion.
The diffusion rate doubles incase concentration difference or surface area are doubled, or the exchange membrane’s thickness is halved.
The exchange surfaces are made up of very thin cell membranes, a state that explains why diffusion is very fast across membranes.