- Amino acids are building blocks of proteins.
- More than 300 amino acids are found in nature but only 20 amino acids are standard and present in protein because they are coded by genes. Other amino acids are modified aminoacids and called non-protein amino acids.
- They are compounds of carbon, hydrogen, oxygen and nitrogen, where the four valences of carbon are attached respectively to an NH2 group, a carboxyl group, an H+ atom and a side chain, which may be polar or non-polar.
- Free amino group is basic and a free carboxyl group is acidic. Hence, an amino acid is both an acid and a base and is called amphoteric.
Acidic, Basic and Neutral amino acids
- Basic amino acids have two amino groups and one carboxyl group. (Diamino-mono carboxylic acid). Hence, the R-group is basic or poitively charged.
E.g. Lysine, Arginine
- Acidic amino acids have one amino group and two carboxyl groups. (Mono-amino dicarboxylic acid). Hence, the R-group is acidic or negatively charged.
E.g. Glutamic acid, Aspartic acid
- Neutral amino acids have one amino group and a carboxyl group (Mono-amino mono-carboxylic acid). E.g. Alanine, Glycine
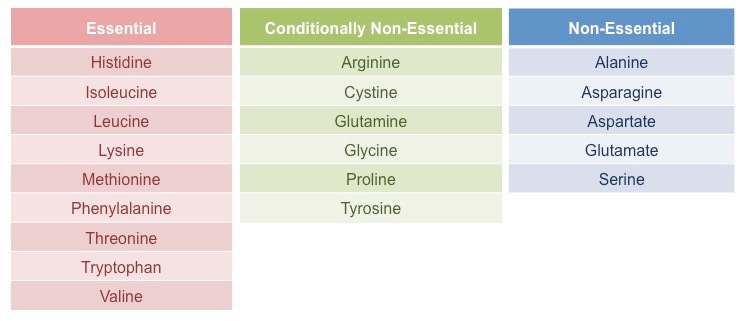
Essential & Non-essential amino acids
- Essential amino acids (9) cannot be synthesized in the body and, therefore, must be present in the diet in order for protein synthesis to occur.
- Non-essential amino acids (11) can be synthesized in the body itself and hence not necessarily need to be acquired through diet.
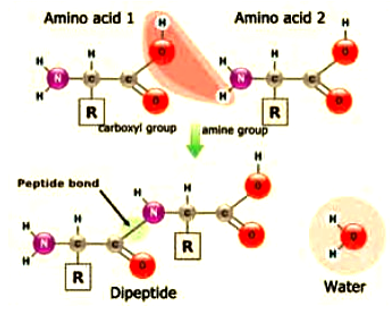
Amino acids are held together by peptide bonds into dipeptides (two amino acid s) oligopeptides (2-10 amino acids) or polypeptides (many amino acids).
Functions of Amino Acids
- Amino acids are the building blocks of proteins.
- Tyrosine is converted into thyroxine, adrenaline and melanin.
- Glycine is involved in the formation of heme (in haemoglobin).
- Tryptophan is needed for the formation of vitamin Nicotinamide (Niacin) and the plant hormone Indole-3-Acetic acid (IAA).
- Amino acids form glucose after the removal of amino group of the carbon chain (Gluconeogenesis).
- Important amines (histamine) are formed by removal of carboxyl group from the aminoacid as carbon dioxide.